Translate this page into:
Use of continuous glucose monitoring in pediatric gastroenterology allows for personalized nutrition support care – Potential for collaboration between pediatric endocrinologists and gastroenterologists

*Corresponding author: Stephanie Oliveira, Department of Pediatric Gastroenterology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, United States. stephanie.oliveira@cchmc.org
-
Received: ,
Accepted: ,
How to cite this article: Hitchcock K, Oliveira S. Use of continuous glucose monitoring in pediatric gastroenterology allows for personalized nutrition support care – Potential for collaboration between pediatric endocrinologists and gastroenterologists. J Pediatr Endocrinol Diabetes 2023;3:34-6.
Abstract
Continuous glucose monitoring (CGM) devices have been approved for the management of patients with diabetes mellitus. Some of the brands available almost completely eliminate the need for finger pricks. We aim to describe our experience with the use of CGM in two patients followed in the gastroenterology clinic due to diseases associated with hypoglycemia and how the information obtained from the devices improved care and outcomes. Data were continuously reviewed through the CGM device remote monitoring system and influenced clinical decisions related to nutrition support. CGMs are now part of standard care for patients with diabetes mellitus. The benefits of these devices and how they can improve the care of patients with disorders typically managed by the gastroenterologist need to be explored. In particular, patients with glycogen storage disorders, fatty acid oxidation defects, and ketotic hypoglycemia can benefit from GCM utilization, allowing for optimal and personalized nutritional care.
Keywords
Continuous glucose monitoring
Glycogen storage disorder
Ketotic hypoglycemia
INTRODUCTION
Continuous glucose monitoring (CGM) devices have been approved for the management of patients with diabetes mellitus. It is a life-changing technology that can significantly impact their quality of life. Some of the brands available almost completely eliminate the need for finger pricks. We aim to describe our experience with the use of CGM in two non-diabetic patients followed in the gastroenterology clinic and how the information obtained from the device improved care and outcomes.
MATERIALS AND METHODS
CGM devices (Dexcom G6 system, Dexcom, Inc., San Diego, CA) were ordered for 2 patients with gastroenterological diseases associated with hypoglycemia [Figure 1]. Data were continuously reviewed through the CGM device remote monitoring system [Table 1] and influenced clinical decisions related to nutrition support. Informed consent was obtained from both patients’ legal guardians.
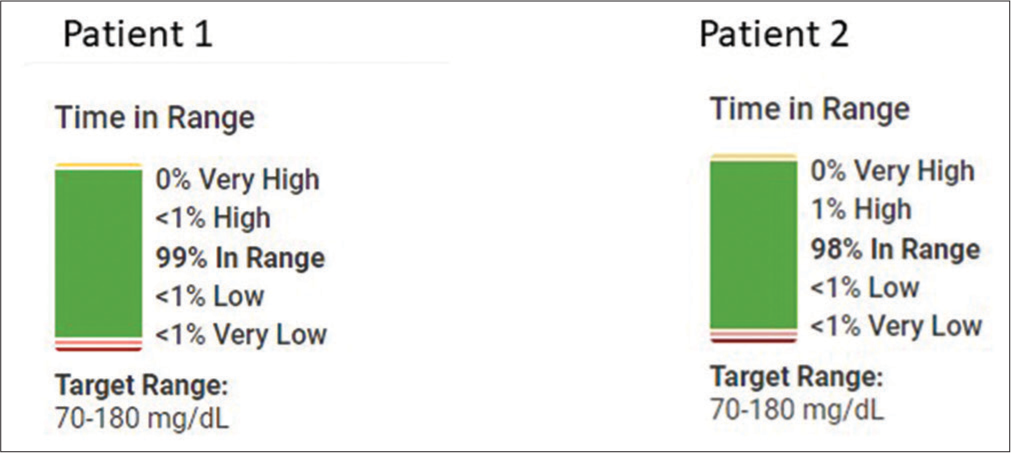
- Both patients have maintained glucose in range for >98% of the time with <1% of low blood sugars in 90 days.
| Patient 1 | Patient 2 | |
|---|---|---|
| Age | 5 years | 3 years |
| Diagnosis | Glycogen storage disease (GSD) type 0 | Autoimmune enteropathy Adrenal insufficiency Ketotic hypoglycemia |
| Nutrition support | Gastric tube | Parenteral nutrition (PN), Gastric tube |
CASE SERIES
Case 1
Patient 1 is a 5-year-old female with a diagnosis of severe hypoglycemic episodes attributed to glycogen storage disorder (GSD) type 0. She was receiving treatment with high amylopectin corn starch by mouth but had a progressive oral aversion, which led to failure to thrive. Eventually, she refused to take corn starch by mouth and the episodes of hypoglycemia became more frequent. Caregivers were measuring spot glucose every 3 h and when she had symptoms of hypoglycemia. We ordered a CGM and were able to identify severe undiagnosed hypoglycemic episodes overnight during fasting. Interestingly, the CGM showed that she did not have a robust response to exogenous glucagon administration during a hospital admission when she experienced severe hypoglycemia, which is consistent with GSD. The CGM helped us determine the schedule to give corn starch during the day, and the frequency of overnight hypoglycemic episodes made us recommend a gastric tube (g-tube) for overnight feeds. The patient now receives overnight continuous formula feeds and the family uses her g-tube for corn starch administration. She is doing well with few sporadic hypoglycemic episodes and has had substantial cognitive development since the nutritional management was optimized, likely related to the significant decrease in hypoglycemic episodes.
Case 2
Patient 2 is a 4-year-old girl with autoimmune enteropathy and iatrogenic adrenal insufficiency. The limiting factor for weaning her off steroids for her autoimmune enteropathy had always been symptomatic ketotic hypoglycemic episodes. She had an extensive medical/genetic workup, which ruled out GSD and congenital disorders of glycosylation. As ketones were present during hypoglycemia, we ruled out fatty acid oxidation disorders. The whole exome sequencing came back normal. Despite the negative workup, the hypoglycemic episodes were severe and symptomatic. We ordered a CGM which helped us identify asymptomatic hypoglycemic episodes allowing the caregiver to prevent the worsening of episodes and admissions to the hospital.
The CGM helped us understand the severity of her hypoglycemia during prolonged fasting. She had a gastric tube for feeds but had a severe intolerance to continuous overnight feeds. This led us to the decision of starting glucose-containing intravenous fluids (IVF) overnight during fasting, which led to improved glycemic control. While the cause of her hypoglycemia remains unclear, she was found to be responsive to diazoxide despite normal insulin levels. We were eventually able to safely discontinue glucose-containing IVF while monitoring her glucose values through CGM as the diazoxide dose was optimized.
DISCUSSION
CGMs are now part of standard care for patients with diabetes mellitus.[1] The benefits of these devices and how they can improve the care of patients with disorders typically managed by the gastroenterologist need to be explored. Patients with GSDs, fatty acid oxidation defects, and ketotic hypoglycemia can benefit from this technology.[2-5] Blood glucose trends obtained from CGM can help guide further workup and management of patients with these conditions. The objective data can guide earlier, targeted interventions before low blood glucose becomes symptomatic.
CGM devices can be an important clinical tool in the management of gastrointestinal diseases associated with hypoglycemia and can positively impact a patient’s quality of life.
Evidence shows that poor metabolic control may increase the risks of complications associated with GSD.[5] Poor metabolic control can occur if patients are unaware of a hypoglycemic episode and make no corrections to glucose measures. CGMs can provide the practitioner and patient with the evidence needed for choosing the optimum medical and/or dietary treatment, which can improve metabolic control.[2-5]
The use of finger pricks with sporadic measurement of glucose can easily miss hypoglycemic episodes, especially in those patients who are asymptomatic to hypoglycemia. This is particularly important during sleep and periods of fasting.[2,5] CGMs can detect glycemic variations that would otherwise go undetected with point-of-care glucose checks, providing reads every 5 min, which can be sent to an application on the patient’s or family member’s cellular device. In addition, CGM monitoring can notify the user when it anticipates a decrease in blood glucose, which can warrant pre-treatment of fast-acting glucose to prevent hypoglycemic episodes.[1] In GSD, unrecognized hyperglycemic episodes, related to overcorrection of hypoglycemia, can also cause significant symptoms and eventually result in health issues such as metabolic syndrome. Utilizing CGM can provide a targeted approach to the management of hypoglycemia unawareness, which can be detrimental to normal development in childhood and increases morbidity related to seizures, coma, and other serious adverse effects of hypoglycemia. The use of CGM can have a significant impact on motivation, compliance, and avoiding disease-related burnout in GSD.[3] Altogether CGM can positively affect patients with GSD, improving energy levels and overall well-being.
CONCLUSION
The use of CGMs in gastroenterology allows for personalized nutrition care of patients with GSDs, fatty acid oxidation defects, and ketotic hypoglycemia, among other conditions.
Quality of life impact
Caregiver 1
“The addition of glycosade®, a g-tube, a CGM, and therapies to address the consequences of years of unmanaged ketotic hypoglycemia gave us a child, we didn’t know existed. She transformed from a kid who couldn’t speak, or sleep, and who had debilitating chronic pain to a thriving and happy kid. She’s starting kindergarten this year alongside her twin, has many friends, and is finally able to do things just for the joy of it like gymnastics and ballet.”
Caregiver 2
“The CGM has saved us many times from finding her passed out in the middle of the night. With the beeping, we are normally able to get in there before it gets really bad. I’m able to sleep a lot better without worrying about if she is dropping constantly. It has definitely had a positive impact on our family as a whole because it gives us more freedom without having to constantly stop and poke her finger throughout the day. Placing and removing the CGM doesn’t even phase her anymore. This has truly been a blessing to our family, especially since we have fought low blood sugar so long before getting it. It has been one less thing to stress about.”
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Continuous glucose monitoring sensors for diabetes management: A review of technologies and applications. Diabetes Metab J. 2019;43:383-97.
- [CrossRef] [PubMed] [Google Scholar]
- Continuous glucose monitoring to diagnose hypoglycemia due to late dumping syndrome in children after gastric surgeries. J Endocr Soc. 2021;5:bvaa197.
- [CrossRef] [PubMed] [Google Scholar]
- Role of continuous glucose monitoring in the management of glycogen storage disorders. J Inherit Metab Dis. 2018;41:917-27.
- [CrossRef] [PubMed] [Google Scholar]
- Usefulness of continuous glucose monitoring for prevention and early detection of hypoglycemia caused by a ketogenic diet and late dumping syndrome. Pediatr Neurol. 2020;105:65-6.
- [CrossRef] [PubMed] [Google Scholar]
- Glycemic control and complications in glycogen storage disease type I: Results from the Swiss registry. Mol Genet Metab. 2019;126:355-61.
- [CrossRef] [PubMed] [Google Scholar]






