Translate this page into:
Treatment of diabetic ketoacidosis with subcutaneous regular insulin in a non-ICU setting is effective and economical: A single-center experience

*Corresponding author: Ahila Ayyavoo, Department of Pediatric Endocrinology and Diabetes, G. Kuppuswamy Naidu Memorial Hospital and Parvathy Clinic, Coimbatore, Tamil Nadu, India. ayyavooahila@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ayyavoo A, Ravikulan A, Palany R. Treatment of diabetic ketoacidosis with subcutaneous regular insulin in a non-ICU setting is effective and economical: A single-center experience. J Pediatr Endocrinol Diabetes 2022;2:50-5.
Abstract
Background:
The mainstay of treatment for diabetic ketoacidosis (DKA) is the correction of dehydration and hyperglycemia with intravenous fluids and intravenous insulin (IVI). Subcutaneous insulin (SCI) has been tried in a few centers in patients with DKA if the blood pH is >7. In general, IVI is preferred over SCI or intramuscular insulin as its onset is rapid and the dose can be titrated based on patient’s varying blood glucose (BG) levels. However, IVI is associated with higher cost of hospitalizations and resource requirements. Thus, SCI could be an alternative to IVI infusion for DKA to reduce these costs and distress, in children during times of resource constraint such as the COVID-19 pandemic and in other resource-limited settings.
Objective:
The objective of the study was to compare the cost and efficacy of SCI therapy versus IVI infusion in the treatment of DKA.
Materials and methods:
A retrospective and cohort study was conducted among children aged 1–15 years admitted with DKA from 2013 to 2014 and treated with IVI and in 2017 treated with SCI at a tertiary hospital. One cohort was treated with IV infusion of regular insulin in intensive care units (ICU) and another cohort was treated with SC regular insulin in pediatric general wards. The main outcomes measured were the overall cost of hospitalization and hours to improvement in the child’s health. The data were analyzed with independent samples t-test with SPSS software.
Results:
Forty-eight patients admitted with 50 episodes of DKA were analyzed. Baseline characteristics of the two groups were similar in age, sex, BG, serum sodium, and HbA1C levels. The proportion of girls to boys was 13:8 (IV insulin group) and 20:9 (SC insulin group); the daily dose of insulin on day 1 of treatment was 1.2:1 unit/kg/day in IVI: SCI groups. The lowest recorded pH was 6.822 (range 6.822–7.154) and 6.831(range 6.831–7.292) in the IVI and SCI groups. The mean pH was 7.00 ± 0.10 and 7.1 ± 0.12, respectively, in IVI and SCI groups (P = 0.02). Episodes of DKA treated with IVI and SCI groups were 21 and 29, respectively. 23% of patients had severe DKA, 52% had moderate DKA, and 25% had mild DKA. The mean time for improvement in the IVI group was 34.95 ± 14.05 h and 17.23 ± 9.85 h in the SCI group (P = 0.001), respectively. The average cost of hospitalization was Rs. 53712 ± 18813 for IVI therapy and Rs. 14369 ± 5768 for SCI (P = 0.000). There were no major complications in the SCI group compared to the IVI group.
Conclusion:
DKA was managed effectively in general wards with SCI therapy with pH not being a limiting factor. Earlier studies have used SCI only in patients with a pH of >7. Therapy with SCI was cost-effective and would be useful in resource-poor settings.
Keywords
Type 1 diabetes
Diabetic ketoacidosis
Subcutaneous insulin therapy
Resource-poor settings
INTRODUCTION
The prevalence of type 1 diabetes mellitus (T1D) is increasing worldwide.[1] The incidence has demonstrated a definite upward trend in the developing countries including India. The current prevalence in India among 5–16-year-old children is 22.2/100,000 population, while it is 3.82/100,000 among 0–5-year-old children in the northern state of Haryana in India.[2] Diabetic ketoacidosis (DKA) remains the most worrisome acute complication in children with T1D.[3] DKA can be potentially fatal when there is a delay in diagnosis or initiation of appropriate treatment. Mortality from DKA is high at 13.2% in India while it is 0.15–0.31% in developed countries.[4,5] Children with T1D could be disadvantaged due to delay in diagnosis which had been noted in 64.7% of children with DKA in India.[6] An improvement in the awareness of T1D and DKA in the developing countries will certainly help improve outcomes including a reduction in mortality.[6]
Use of intravenous insulin infusion (IVI) along with appropriate fluid management remains the cornerstone of therapy for children with DKA and they are often managed in intensive care unit (ICU) or high dependency units (HDUs).[7] Although subcutaneous insulin (SCI) has been suggested for the management of DKA, the data on this alternate form of therapy are sparse.[7,8] Before the year 2000, non-intravenous route had been used for the treatment of DKA.[9]
Currently, IVI is preferred over SCI as onset of action is rapid, and the dose of insulin can be titrated rapidly based on blood glucose (BG). Some centers have used rapid acting insulin analogs every 1 or 2 h subcutaneously in the management of DKA in adults and children with a pH more than 7.[10-13] While the children on IVI recovered earlier than the children on SCI, both the groups recovered without complications.[10] Pediatric ICUs and trained personnel are available in very few centers in India.
The average cost of therapy for children with an episode of DKA from 2004 to 2009 in the USA was $7142 while it has not been quantified in India.[3] This is an enormous financial burden on the family and public health system. Simplifying therapy and reducing cost could be useful in improving outcomes of children with DKA in resource-poor countries, such as India.
With the above facts, the protocol for the management of DKA was shifted from IVI to SCI from 2014 at G. Kuppuswamy Naidu Memorial Hospital (GKNMH) in Coimbatore, India. The care of these children was shifted to the general ward from the ICU to reduce costs.
This study aimed to evaluate the cost and outcomes of IVI versus SCI in the management of DKA by a retrospective and cohort study.
MATERIALS AND METHODS
This was a retrospective and cohort study of patients admitted to the pediatric ward or pediatric ICU at GKNMH and treated for DKA during 2013–2014 with IVI and with SCI in 2017. The study had been approved by the Institutional Ethics Committee. Data were extracted from the hospital records.
Blood glucose was checked in all children with symptoms of polyuria, polyphagia, weight loss, or breathlessness. Children with the clinical features of dehydration, tachypnea, tachycardia, smell of acetone in breath, nausea/ vomiting, abdominal pain, drowsiness, alteration or loss in consciousness, and biochemical criteria of a BG >200 mg/ dL and acidosis (pH <7.3 or serum bicarbonate <15 mEq/L) were diagnosed as DKA.[8] Severity of DKA was classified based on the acidosis according to International Society for Pediatric and Adolescent Diabetes (ISPAD) criteria: Mild = venous pH 7.20–7.29 or serum bicarbonate 10 to ≤15 mEq/L; moderate = venous pH 7.10–7.19 or serum bicarbonate 5–≤9.9 mEq/L; severe = venous pH <7.1 or serum bicarbonate <5 mEq/L.[8] The protocol outlined in the clinical pathway in [Figure 1] was used in the management of DKA.
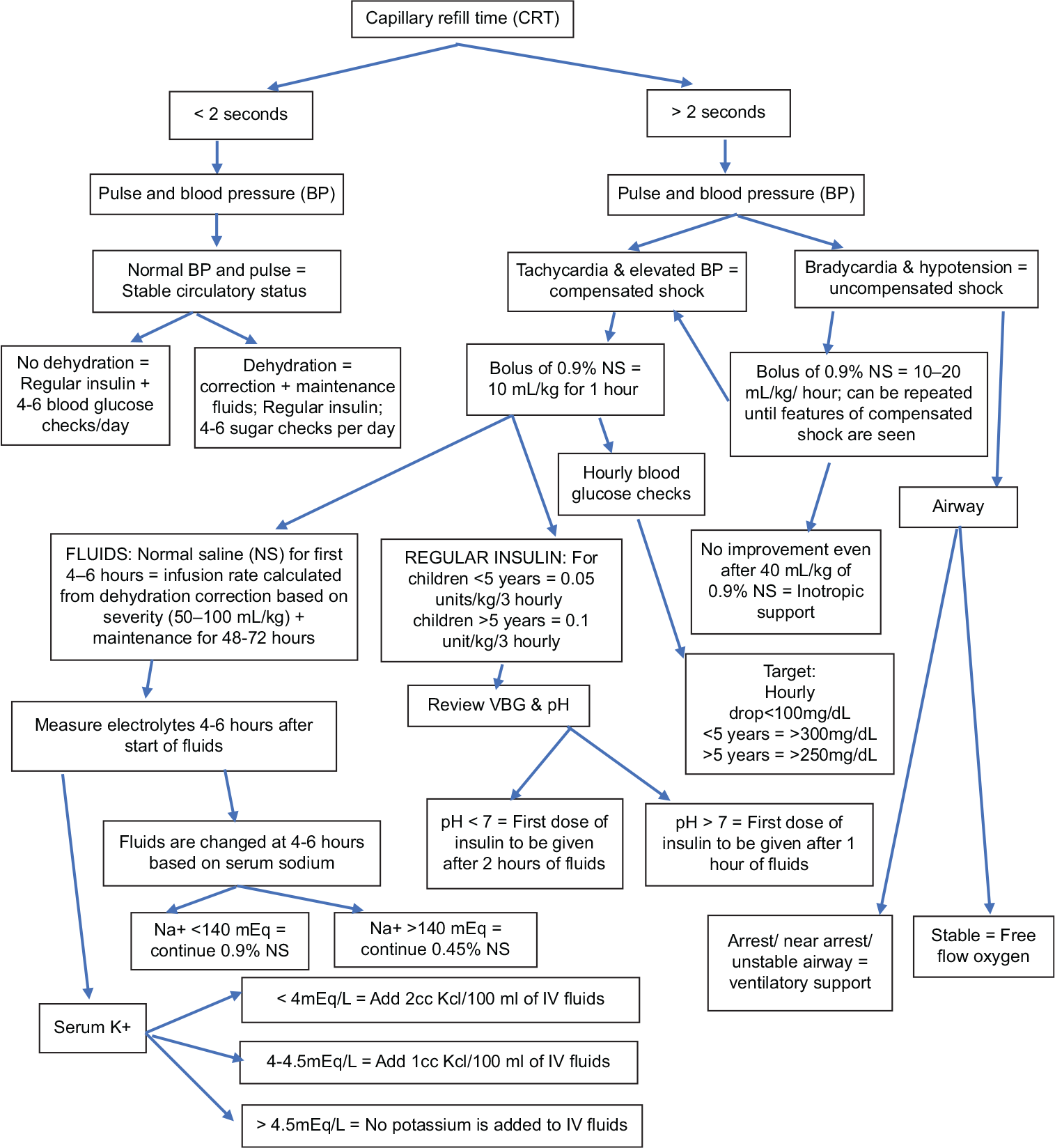
- Subcutaneous insulin for the treatment of pediatric diabetic ketoacidosis.
The children were assessed as per Pediatric Advanced Life Support protocol. Two peripheral intravenous lines were established and samples of blood were drawn for venous blood gas, serum electrolytes, urea, creatinine, HbA1C, blood counts, ketones, and C-peptide. Children with signs of DKA were classified into three groups based on capillary refill time (CRT), pulse rate, and blood pressure (BP): Stable circulatory status, compensated shock, or uncompensated shock. Children with unstable airway, needing cardiopulmonary resuscitation, were immediately shifted to pediatric intensive care unit with ventilatory support, fluid correction, and IVI infusion as per ISPAD protocol.[8]
The treatment of children was shifted to general wards since 2015, if they did not need ventilator support and if they responded to the mother’s voice. Two of the patients in IVI cohort needed ventilatory support. The management goals were based on the ISPAD protocol and gradual replacement of fluids was the most crucial target.[8]
Fluids were administered to both groups following the same principles described below. A 10 ml/kg of 0.9% of normal saline (NS) was given as a bolus over 1 h in children with shock. More boluses of NS were given if required in severe shock. Fluid deficit was replaced over 72 h in children <5 years and over 48 h in children >5 years. Maintenance fluids were calculated based on Holliday-Segar formula for children weighing <30 kg and 1500 mL/m2/day for children >30 kg. Fluid calculation for dehydration correction was calculated as 5% or 50 mL/kg (capillary refill time [CRT] >2 seconds and tenting skin) and as 10% dehydration or 100 mL/kg (poor peripheral pulses or hypotension or oliguria) or 7.5% dehydration for features which were between these two states.
Treatment of DKA was shifted to SCI from IVI infusion gradually in 2015 to help families from poor socioeconomic status. As the team’s experience in managing DKA with SCI grew, this became the primary mode of the treatment of DKA. One cohort treated with IV infusion of regular insulin in ICU and another cohort treated with SC regular insulin in pediatric general wards were compared. All children presenting with DKA to the hospital during the study period of 2013–14 and 2017 were analyzed. Only one child was excluded as the child had presented with cardiorespiratory arrest and multiple cerebral infarcts at a hospital 300 km away and had been treated, stabilized, and shifted on a transport ventilator.
Insulin infusion in the IVI cohort was started after an hour of fluids at the rate of 0.05 units/kg/h for children under the age of 5 years and at the rate of 0.05–0.1 units/kg/h for children older than 5 years of age.
The first dose of regular insulin in DKA in the SCI was given subcutaneously after 60 min of fluids for pH >7 and 90–120 min for pH <7. The dose of regular insulin was 0.05 units/kg for the first dose in pH <7. Doses were repeated every 2–3 h at 0.05–0.1 unit/kg thereafter. Serum electrolytes were repeated after 4–6 h and fluids were changed as NS/half-NS based on sodium. Potassium was added as shown in [Figure 1]. Bicarbonate or phosphate infusion was not used in any of the patients in this study. Time to recovery was calculated as the first instance of taking food.
The cost of treatment was calculated by including cost of clinical pathology, microbiology, biochemistry, diet, pharmacy, bed, nursing charges, doctors’ fees, surgical materials, and other incidental expenditure. These were corrected to the charges of the final year of the study period so that the costs across the years were comparable.
Statistical analyses
Two groups were analyzed: Group 1 was treated with IVI and group 2 with SCI. The primary outcomes measured were the overall cost of hospitalization and hours to improvement in the child’s health. Data were analyzed with independent samples t-test with SPSS software.
RESULTS
Fifty episodes of DKA in 48 patients were analyzed after excluding a child with multiple cerebral infarcts. About 29 episodes were treated with SCI and 21 episodes of DKA with IVI.
Baseline characteristics [Table 1] of both groups were similar for age (P = 0.68), sex (P = 0.62), HbA1C at presentation (P = 0.91), serum sodium (P = 0.24), and BG (P = 0.43). Children under 3 years in the IVI cohort were 4, while it was 1 in the SCI cohort. Metabolic acidosis parameters, pH (P = 0.04), serum potassium (P = 0.00), and serum bicarbonate (P = 0.03), were lower in the IVI cohort. The cost for treatment for the entire hospitalization for DKA using SCI was Indian Rupees (INR) 14,368.94 ± 5767.48, while it was INR 53,712.04 ± 18,813.40 when IVI (P = 0.00004) was used [Figure 2]. Treatment of DKA with IVI in the ICU and HDU was 3.7 times costlier. Children treated with SCI recovered within 17.23 ± 9.86 h while those treated with IVI took longer to recover at 34.95 ± 14.05 h (P = 0.00137) [Figure 3].
| Parameter | IVI group | SCI group | P-value |
|---|---|---|---|
| Number of DKA episodes | 21 | 29 | |
| Girls: boys | 13:8 | 20:9 | 0.62 |
| Age (years) | 7.52±3.91 | 8.05±3.56 | 0.68 |
| Blood glucose (mg/dL) |
440.05±110.62 | 417.72±73.73 | 0.43 |
| HbA1C at presentation (%) | 12.2±1.09 | 12.88±1.28 | 0.91 |
| pH | 7.00±0.11 | 7.09±0.12 | 0.04 |
| Bicarbonate (mEq/L) | 4.41±1.42 | 7.10±2.89 | 0.03 |
| Sodium (mEq/L) | 134.56±6.35 | 135.88±5.7 | 0.24 |
| Potassium (mEq/L) | 4.01±0.75 | 4.91±0.83 | 0.00 |
| Lowest recorded pH | 6.822 | 6.831 |
IVI: Intravenous insulin, SCI: Subcutaneous insulin, DKA: Diabetic ketoacidosis
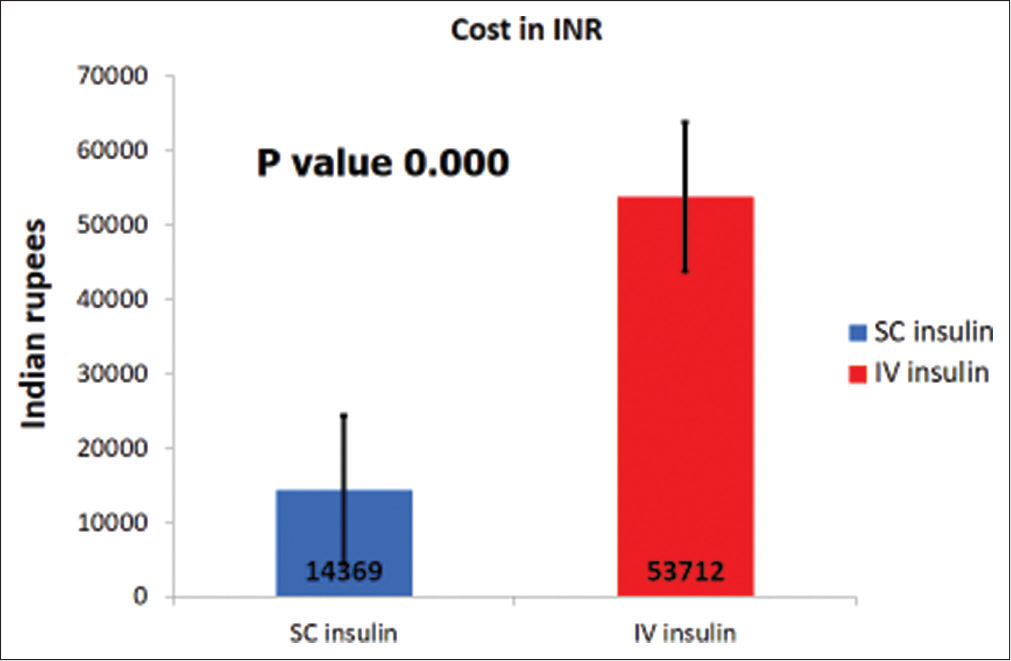
- Cost of therapy.
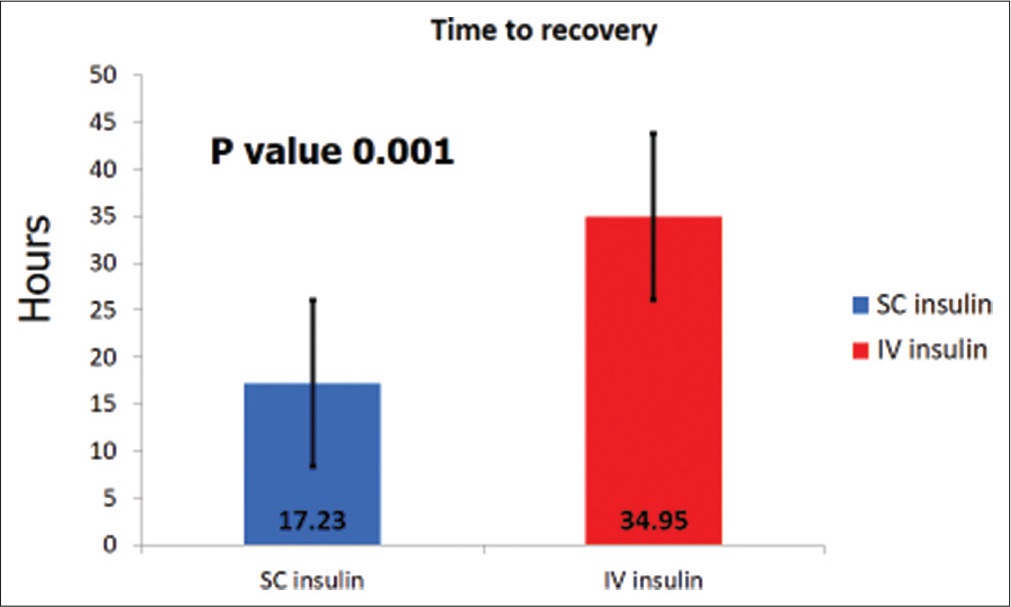
- Time to recovery from DKA.
The range of pH in the group treated with SCI was 6.831–7.292 and in children treated with IVI was 6.822–7.154. The time to recovery in the child with the lowest pH on SCI was 10 h and 35 h in the child with the lowest pH on IVI infusion. Around 47.4% of patients treated with SCI had an arrival pH of <7.1 and 26.4% had a pH of <7. The mean dose of insulin used in children on SCI therapy was 1 unit/kg/day and 1.2 unit/kg/day in children on IVI infusion. There were no clinically recorded cases of cerebral edema or hypoglycemia in the study population. There was no mortality during the study period.
DISCUSSION
The cost of treatment for children with DKA using subcutaneous regular insulin was 3½ times cheaper than the expenses incurred with IVI infusion. The time taken for recovery from DKA for children treated with SCI was half the time duration taken for improvement in children treated with IVI. The age, sex, HbA1C, and BG at presentation for children treated with SCI were similar to those with IVI. However, the pH and serum bicarbonate were slightly lower in children treated with IVI.
This is the first study from India in the recent past that has evaluated the use of subcutaneous regular insulin in the treatment of DKA in children. An earlier study from Brazil demonstrated the usefulness of SCI analog in treating DKA in children.[10] While lispro was used in the study by Manna et al, regular insulin was used in our study even in children with severe DKA.[10] The subjects involved in the study from Brazil had moderate DKA with a pH of >7.1, while children at presentation in our study had even more severe acidosis with a pH of <7.1 in 47.4% of children on SCI.[10] Bicarbonate infusion or phosphate supplementation was not given to any patient in our study, in contrast to the study by Manna and colleagues. The population in our study was younger at 7.5–8 years of age compared with the group from Brazil aged 11.3–12.1 years.[10] The same group published their protocol mentioning the initiation of mechanical ventilation in patients with shock, while our protocol used ventilation only in two children with the unstable airway.[13] The protocol at the current study center followed a similar protocol with the initial concentration being on appropriate fluid administration followed by insulin dosing.
Cohen and colleagues also have used subcutaneous regular insulin to treat pediatric DKA in Israel.[14] The time for resolution of DKA and the absence of severe complications were similar to our study. However, the study had used SCI only in children with a pH >7 while 26.4% of our SCI cohort had a pH of <7. The mean age group of children in the Israeli study was 3 years older than the study population in our group.[14]
Another study that evaluated the use of SCI for treating DKA involved adult population with moderate DKA at a venous pH of 7.14 to 7.15.[12] Umpierrez et al. used a higher loading dose than the subsequent one in the above study.[12] We used a lower dose at the start of therapy and increased the subsequent dosing to avoid the risk of cerebral edema that may ensue with a rapid drop in the BG.
Most experts worldwide prefer IVI over SCI due to the delay in the onset of action and the prolonged duration of action.[12] The reason may be due to lack of trained staff on the use of SCI in the general wards. Hence, IVI for treating DKA is generally carried out in ICU or HDUs. This increases the overall cost of therapy, which would be a substantial limiting factor in several countries with resource limitations. The average cost of treatment of a single episode of DKA in an ICU in the USA was US$ 11,000 in 2004 (equivalent to INR 498,520 in 2004).[12] Our present study demonstrated that using SCI for DKA in regular general wards was feasible and drastically reduced the cost of therapy from INR 53,712 for IVI to INR 14,368 for SCI. The cost of treatment between IVI and SCI has never been assessed earlier in India.
The time to recovery while using SCI therapy was half the time taken to recover with IVI therapy. Our study demonstrated faster recovery from DKA with SCI while other studies using have not shown any advantage in the time to recovery.[12]
Children with severe DKA with a pH of 6.8 could be treated successfully with SCI therapy in this study. Earlier studies using SCI for DKA considered a pH of <7 as a limiting factor for using SCI. The time for recovery was 10 h for the child with the lowest pH in SCI compared to 35 h for the child with the lowest pH in the IVI group. Thus, this study has demonstrated the use of SCI even for pH <7.
Although the pH and bicarbonate levels were lower in the group on IVI therapy, the total daily dose of insulin used in either group was similar. There was no difference in the doses of insulin used during treatment of DKA in the SCI or IVI therapy. Recovery was not hindered by a reduction in the frequency of investigations.
It would be important to note if the SCI therapy would also be effective in other centers. The use of SCI for treating DKA would be a good option for reducing costs in countries with severe limitations to investigation capability, staff availability, and economy. In times of extreme source restraints such as the COVID-19 pandemic, using SCI therapy could be a practical option.
The limitations of this study are the use of oral intake as a surrogate marker for resolution of acidosis being subjective and not the approved endpoint mentioned in the current guidelines, acidosis in the children being treated with IVI being more severe, retrospective collection of data and evaluation, small sample size, non-homogeneous cohorts, groups not being compared with a well-drawn randomized design of the study, and the experience being from a single center.
Although there are several advantages of SCI therapy, it should be emphasized that for achieving the best results, the medical and nursing staff in the general wards do require specific knowledge and skills, and training for handling these children with DKA. The health-care staff must provide dedicated care with constant monitoring of the child’s clinical parameters during hospitalization.
CONCLUSION
This study has proven the utility of SCI therapy in the management of DKA. SCI therapy is an acceptable mode of treatment in the management of DKA in under-developed and developing countries with poor resources. This study reiterates the fact that SCI resulted in faster recovery in children even while being safely managed in the general medical wards under appropriate nursing supervision with the arrival pH not being a limiting factor. This would be helpful in scenarios where ICU facilities are sparse.
Declaration of patient consent
This retrospective study only involved collection of data from case records with no identification details. Hence, Institutional Ethics Committee consent was obtained without a need for patient’s consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Incidence and trends of childhood Type 1 diabetes worldwide 1990-1999. Diabet Med. 2006;23:857-66.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of Type 1 diabetes mellitus in Karnal district, Haryana state, India. Diabetol Metab Syndr. 2010;2:14.
- [CrossRef] [PubMed] [Google Scholar]
- Rates of diabetic ketoacidosis: International comparison with 49,859 pediatric patients with Type 1 diabetes from England, Wales, the US, Austria, and Germany. Diabetes Care. 2015;38:1876-82.
- [CrossRef] [PubMed] [Google Scholar]
- Diabetic ketoacidosis: Predictors of outcome in a pediatric intensive care unit of a developing country. Pediatr Crit Care Med. 2004;5:427-33.
- [CrossRef] [PubMed] [Google Scholar]
- International Society for pediatric and adolescent diabetes, ISPAD clinical practice consensus guidelines 2014. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes. 2014;15:154-79.
- [CrossRef] [PubMed] [Google Scholar]
- Delayed diagnosis of diabetic ketoacidosis in children-a cause for concern. Int J Diabetes Dev Ctries. 2015;35:66-70.
- [CrossRef] [Google Scholar]
- Diabetic ketoacidosis in children and adolescents. Indian J Endocrinol Metab. 2015;19:S55-7.
- [CrossRef] [PubMed] [Google Scholar]
- ISPAD clinical practice consensus guidelines 2018: Diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. 2018;19:155-77.
- [CrossRef] [PubMed] [Google Scholar]
- Management of hyperglycemic crises in patients with diabetes. Diabetes Care. 2001;24:131-53.
- [CrossRef] [PubMed] [Google Scholar]
- Subcutaneous use of a fast-acting insulin analog: An alternative treatment for pediatric patients with diabetic ketoacidosis. Diabetes Care. 2005;28:1856-61.
- [CrossRef] [PubMed] [Google Scholar]
- Subcutaneous lispro and intravenous regular insulin treatments are equally effective and safe for the treatment of mild and moderate diabetic ketoacidosis in adult patients. Int J Clin Pract. 2006;60:429-33.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of diabetic ketoacidosis with subcutaneous insulin aspart. Diabetes Care. 2004;27:1873-8.
- [CrossRef] [PubMed] [Google Scholar]
- Alternative management of diabetic ketoacidosis in a Brazilian pediatric emergency department. Diabetol Metab Syndr. 2010;2:41.
- [CrossRef] [PubMed] [Google Scholar]
- Subcutaneous regular insulin for the treatment of diabetic ketoacidosis in children. Pediatr Diabetes. 2017;18:290-6.
- [CrossRef] [PubMed] [Google Scholar]







