Translate this page into:
Hypopituitarism secondary to pediatric traumatic brain injury – A need for active vigilance

*Corresponding author: Akanksha Chirag Parikh, Department of Paediatric Endocrinology, Kokilaben Dhirubhai Ambani Hospital, Mumbai, Maharashtra, India. gandhi.akanksha@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Parikh AC, Deorukhkar PR. Hypopituitarism secondary to pediatric traumatic brain injury – A need for active vigilance. J Pediatr Endocrinol Diabetes. 2024;4:101-4. doi: 10.25259/JPED_39_2024
Abstract
Hypopituitarism, a known sequelae of pediatric traumatic brain injury (TBI), is often overlooked, especially in acute settings where more dynamic issues warrant the clinician’s attention. Pituitary dysfunction following pediatric TBI usually develops early and is often transient. However, a long-term follow-up is required to monitor the ongoing needs of hormonal replacement therapy as well as to identify the onset of new hormonal deficiencies, which can develop many years after the brain injury. This case describes the clinical course of an infant who suffered a fall and developed multiple skull fractures. The child required admission to intensive care and developed hypopituitarism (cortisol, thyroid, vasopressin, and growth hormone) secondary to severe TBI. The clinical (refractory shock and polyurea) and radiological red flags (empty sella) can assist in predicting the development of pituitary dysfunction in such cases.
Keywords
Traumatic brain injury
Hypopituitarism
Pediatric
Permanent
INTRODUCTION
Pituitary dysfunction can occur in children who have suffered a traumatic brain injury. Although reported to be mostly early and transient, it can persist in some children and can even develop many months or years after the traumatic event. We report a young infant who developed early and persistent hypopituitarism because of a severe traumatic brain injury, outlining the importance of active vigilance in the acute setting as well as in the long-term.
CASE REPORT
A 10-month-old female infant, previously healthy and of foreign origin, suffered a fall from an almirah door while sleeping, resulting in bilateral ear, nose, and mouth bleeding and left eye medial strabismus. Before the incident, her perinatal period was uneventful, developmental milestones were on track, and growth was normal. Born to non-consanguineous parents, she also had a healthy 3-year-old brother.
On examination, she exhibited altered consciousness (Glasgow Coma Scale ([GCS] 3/15), gasping respiration, and circulatory shock. Initial tests showed anemia, transient mild hypokalemia (3 meq/L), and reactive leukocytosis. A brain computed tomography (CT) scan revealed over ten bilateral skull fractures, including a comminuted sphenoid bone fracture extending to the sella turcica and small bilateral subdural and epidural hemorrhages. She required mechanical ventilation and intensive care unit admission. High-dose intravenous hydrocortisone (5 mg every 6 h) was started due to inotrope-resistant hypotension. Conservative management for the head trauma was advised by otolaryngology and neurosurgery teams. On the 7th day, she developed meningitis symptoms, leading to the cessation of hydrocortisone therapy. In the following days, she experienced multiple vomiting episodes, hypotension, asymptomatic hypoglycemia, and intermittent polyuria with a maximum urine output of 10 mL/kg/h, along with hyponatremia (serum sodium: 131–133 meq/L) and normokalemia. Morning serum cortisol was 1.1 µg/dL (5–23 µg/dL) with adrenocorticotropic hormone (ACTH) <5 pg/mL (9–52 pg/mL). Brain magnetic resonance imaging (MRI) indicated diffuse axonal injury and leptomeningeal enhancement without pituitary abnormalities; adrenal ultrasonography showed no hemorrhage. Suspecting adrenal insufficiency due to high-dose glucocorticoid therapy, oral hydrocortisone was continued at a physiological dose of 7.5 mg/day in three divided doses. Consequently, hypoglycemia, hypotension, and hyponatremia improved; serum sodium rose to 138 meq/L, and oral intake improved. After 3 weeks, she was discharged with a GCS score of 13/15 and persistent left lateral rectus palsy, on oral hydrocortisone, levetiracetam, lansoprazole, and cotrimoxazole, with follow-up evaluations planned.
Two weeks post-discharge, the infant was in good health except for increased urinary output requiring frequent diaper changes, as reported by the mother. Anthropometric measurements were weight 8.4 kg (–0.31 standard deviation [SD]), length 75 cm (0.88 SD), and head circumference 42.8 cm (–1.32 SD). A subsequent serum cortisol test (following the discontinuation of the previous evening and morning dose) showed low levels (0.62 µg/dL). Hypopituitarism secondary to traumatic brain injury (TBI) was considered. Biochemical tests suggested central hypothyroidism (free thyroxine [T4]: 0.27 ng/dL and thyroid-stimulating hormone [TSH]: 1.47 IU/L) and diabetes insipidus (serum sodium: 146 meq/L, serum osmolality: 300 mosm/kg, and urine osmolality: 200 mosm/kg). An MRI of the pituitary gland revealed an empty sella and an absence of the posterior pituitary bright spot [Figure 1]. A review of prior CT and MRI images confirmed the empty sella since admission.
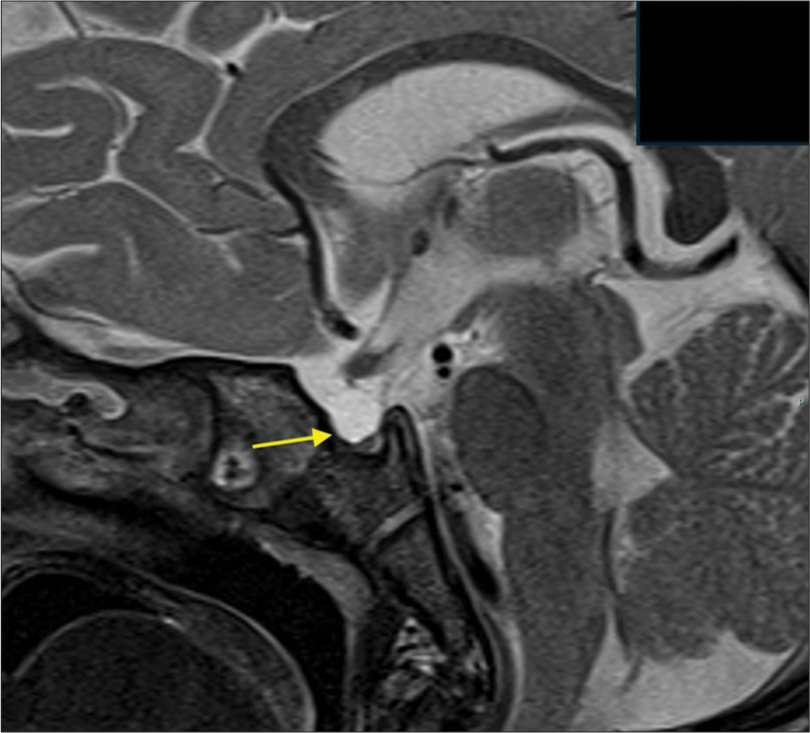
- T2-weighted image of the sagittal view of the hypothalamic-pituitary gland showing an outpouching of cerebrospinal fluid (Yellow arrow) in the sella suggestive of empty sella syndrome.
The child was treated with physiological doses of hydrocortisone, 37.5 µg daily levothyroxine, and 25 µg desmopressin. At a 3-month follow-up, the child was asymptomatic with adequate growth, on reduced daily doses of desmopressin (20 µg) due to hyponatremia and levothyroxine (25 µg) due to elevated T4 levels. However, at a 1-year post-TBI physical consultation, the child exhibited growth failure with a length of 77 cm (–1.89 SD), height velocity of 2 cm/year, and a weight of 10.2 kg (–0.35 SD). Tests at another facility showed a maximum growth hormone response to glucagon stimulation of <0.1 µg/dL. The child required ongoing daily doses of 25 µg desmopressin, 15 mg/m2 hydrocortisone, and 25 µg levothyroxine, and recombinant growth hormone therapy was recommended.
DISCUSSION
Hypopituitarism is a significant yet often overlooked consequence of moderate and severe TBI. Most cases manifest within the first 3–6 months, but new hormonal deficiencies can emerge years later.[1] Although pediatric TBI data are limited, an overall prevalence of 20% has been reported.[2] This case highlights the need for vigilance regarding hypopituitarism following significant TBI.
Proposed mechanisms for pituitary dysfunction post-TBI include primary injury to the hypothalamic-pituitary region, particularly after a skull-base fracture or shearing of hypophyseal vessels, leading to cerebral edema, ischemia, and inflammation.[3] The development of pituitary antibodies might also explain hypopituitarism occurring years post-TBI and could serve as a predictive tool.[2,4]
In acute TBI, hypocortisolism and diabetes insipidus can significantly affect clinical outcomes and require active surveillance. Symptoms such as nausea, vomiting, hypoglycemia, hypotension, polyuria, and clinical deterioration, alongside electrolyte imbalances like hypo- or hypernatremia, suggest possible pituitary dysfunction, as seen in our case. Although no longer recommended, glucocorticoids were traditionally administered to children with severe TBI to reduce cerebral edema and improve neurological outcomes;[5] they may also be indicated for inotrope-resistant shock. Hypocortisolism may be mistakenly attributed to iatrogenic adrenal insufficiency from high-dose glucocorticoids, necessitating close follow-up for hydrocortisone supplementation. Bilateral adrenal hemorrhage from high-impact blunt trauma, especially in multisystem organ injury, should be considered, warranting ultrasonography or contrast-enhanced CT of the adrenal glands in suspicious cases.[6] Difficult labor and non-accidental injury can cause both TBI and adrenal hemorrhage in neonates.[7]
Growth hormone deficiency, followed by gonadotropin deficiency, is the most common hormonal issue in children after TBI.[7] Therefore, regular monitoring of growth and puberty in these children is crucial for early intervention. Conversely, a history of TBI should be investigated in children with unexplained short stature and delayed puberty. Central precocious puberty has also been noted in studies tracking children post-moderate to severe TBI.[2,9-11] While ACTH and TSH deficiencies are rare, they can lead to severe consequences if undiagnosed. Pituitary dysfunction may sometimes be subclinical, shown by normal growth velocity despite low insulin-like growth factor 1 or insulin-like growth factor-binding protein 3 and poor response to stimulation tests.[10,12,13] Obesity in these children can complicate diagnosis by either affecting growth hormone responses or allowing growth without growth hormone, as seen in hypothalamic obesity cases.[3,11] Monitoring the growth pattern is essential to determine the correct scenario. Our patient developed early hypocortisolism, central hypothyroidism, and diabetes insipidus, with growth failure and growth hormone deficiency diagnosed later, underscoring the need for vigilant growth monitoring.
Certain risk factors, though not fully validated, may predict hypopituitarism in TBI-affected children, such as moderate-to-severe head trauma (GCS score 9–11 and <9, respectively).[3] Other risk factors include raised intracranial pressure, diffuse axonal injury, basilar skull fracture, diffuse cerebral edema, and intracranial hematoma.[7] Our patient had several risk factors, including a low GCS score at admission, sphenoid bone fracture, and diffuse axonal injury on MRI, highlighting the need for vigilant monitoring for hypopituitarism.
Common MRI changes of the pituitary gland in pediatric TBI include acute edematous enlargement followed by long-term atrophy and perfusion defects.[2] In our case, the initial neuroimages showed an empty sella, suggesting either congenital empty sella syndrome with hypopituitarism triggered by TBI or acute symptomatic empty sella syndrome post-trauma. The latter is documented in adults but not in children.[14] Thus, it is likely our patient had an undiagnosed asymptomatic empty sella revealed by TBI.
Early-onset pituitary dysfunction in pediatric TBI is mostly transient, recovering spontaneously within 3–6 months due to higher neuroplasticity in children. Late-onset hormonal deficiencies, although rare, tend to be persistent.[1] Our patient showed tapering of hormone doses early on, but the continued need for therapy after 1 year, along with MRI findings, suggests permanent hypopituitarism.
Determining which children with TBI need evaluation for hypopituitarism remains challenging, with no consensus guidelines on the population, timing, frequency, or screening methods. Moderate-to-severe TBI, or mild TBI with risk factors, can be screened for ACTH deficiency through early morning cortisol measurements at admission and discharge.[3] Subsequent clinical monitoring of growth, puberty, and thyroid function should be quarterly for 1 year and then annually for 5 years.[3] Growth hormone and gonadotropin deficiency should be evaluated if growth retardation or delayed puberty is observed.
CONCLUSION
In conclusion, hypopituitarism after pediatric TBI commonly emerges in the acute phase and within the first few months. Growth hormone and gonadotropin deficiencies may only become apparent with close monitoring of growth and puberty. Conversely, a history of TBI should be considered in children with short stature or delayed puberty. Although often transient, persistent endocrine dysfunction can occur, especially if onset is beyond 6 months to a year post-TBI. High clinical suspicion is crucial, especially in moderate-to-severe TBI cases, to enhance clinical outcomes both acutely and long term.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- An approach to traumatic brain injury-related hypopituitarism: Overcoming the pediatric challenges. Diagnostics (Basel). 2023;13:212.
- [CrossRef] [PubMed] [Google Scholar]
- Traumatic brain injury: Is the pituitary out of Harm's way? J Pediatr. 2011;159:686-90.
- [CrossRef] [PubMed] [Google Scholar]
- Update of endocrine dysfunction following pediatric traumatic brain injury. J Clin Med. 2015;4:1536-60.
- [CrossRef] [PubMed] [Google Scholar]
- Antipituitary antibodies after traumatic brain injury: Is head trauma-induced pituitary dysfunction associated with autoimmunity? Eur J Endocrinol. 2008;159:7-13.
- [CrossRef] [PubMed] [Google Scholar]
- Management of severe traumatic brain injury in pediatric patients. Front toxicol. 2022;4:910972.
- [CrossRef] [PubMed] [Google Scholar]
- Blunt adrenal gland trauma in the pediatric population. Asian J Surg. 2011;34:103-10.
- [CrossRef] [PubMed] [Google Scholar]
- Traumatic brain injury induced hypopituitarism in children and adolescents. Pediatr Health. 2009;3:283-91.
- [CrossRef] [Google Scholar]
- Occurrence of pituitary dysfunction following traumatic brain injury. J Neurotrauma. 2004;21:685-96.
- [CrossRef] [PubMed] [Google Scholar]
- Pituitary function in paediatric survivors of severe traumatic brain injury. Arch Dis Child. 2008;93:133-7.
- [CrossRef] [PubMed] [Google Scholar]
- Measurement of height velocity is an useful marker for monitoring pituitary function in patients who had a traumatic brain injury. Pituitary. 2013;16:499-506.
- [CrossRef] [PubMed] [Google Scholar]
- Permanent hypopituitarism is rare after structural traumatic brain injury in early childhood. Clin Endocrinol Metab. 2012;97:599-604.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of pituitary function after traumatic brain injury in childhood. Clin Endocrinol (Oxf). 2010;73:637-43.
- [CrossRef] [PubMed] [Google Scholar]
- Pituitary dysfunction after traumatic brain injury in children: Is there a need for ongoing endocrine assessment? Clin Endocrinol (Oxf). 2013;79:853-8.
- [CrossRef] [PubMed] [Google Scholar]
- High frequency of empty sella, with gender differences, in the early neuroradiology evaluation of patients with traumatic brain injury. A prospective study. J Clin Transl Endocrinol. 2019;15:54-61.
- [CrossRef] [PubMed] [Google Scholar]







