Translate this page into:
Efficacy and safety of aromatase inhibitors in the management of idiopathic short stature: A meta-analysis

*Corresponding author: Deep Dutta, Department of Endocrinology, Center for Endocrinology Diabetes Arthritis and Rheumatism (CEDAR) Superspeciality Healthcare, Dwarka, New Delhi, India. deepdutta2000@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Dutta D, Agrawal R, Joshi A, Sharma M. Efficacy and safety of aromatase inhibitors in the management of idiopathic short stature: A meta-analysis. J Pediatr Endocrinol Diabetes. 2024;4:21-30. doi: 10.25259/JPED_42_2023
Abstract
Objectives:
Data are scant on the efficacy and safety of aromatase inhibitors (AIs) in idiopathic short stature (ISS). We undertook this meta-analysis to address this knowledge gap.
Material and Methods:
Electronic databases were searched for randomized control trials (RCTs) involving children with ISS receiving AIs compared to placebo/active comparator. The primary outcome was changes in predicted adult height (PAH). Secondary outcomes were alterations in bone age, puberty hormones, and side effects.
Results:
One-thousand three-hundred and eighty articles were reviewed, from which 4 RCTs which fulfilled all criteria were analyzed (one in the active control group [ACG] having growth hormone [GH] as an active comparator; three in the passive control group having placebo as controls). AIs were superior to placebo with regards to improvement in PAH (mean difference, MD 4.62 cm [95% confidence interval, CI: 4.02–5.23]; P < 0.01; I2 = 0%), bone-age progression (MD −0.61 years [95% CI: −0.87–−0.35]; P < 0.01; I2 = 0%) and height-standard deviation score improvement (MD 0.43 [95% CI: 0.33–0.53]; P < 0.01; I2 = 88%). No increased adverse events and spinal deformities were noted with AIs.
Conclusion:
AIs are safe and effective for improving height and pubertal outcomes in ISS. There remains scope for using AIs and GH together in ISS to have a synergistic impact on height outcomes.
Keywords
Letrozole
Meta-analysis
Safety
Constitutional delay in growth and puberty
Short stature
INTRODUCTION
Idiopathic short stature (ISS) has been defined as the presence of a height more than two standard deviations (SD) below the corresponding mean height for chronologic age, sex, and population in a child with normal birth size and normal body proportions, and without evidence of any systemic, endocrine, nutritional, or chromosomal abnormalities.[1] Constitutional delay in growth and puberty (CDGP) and familial short stature (FSS), the two most common causes of “normal-variant” short stature, need to be ruled out before diagnosing ISS. Hence, the diagnosis of ISS is often a diagnosis of exclusion. Due to the nature of its definition, ISS includes a heterogeneous group of children with variable phenotypes and genotypes and is perhaps one of the most common causes of short stature.[2] With the use of different genetic techniques such as copy number variant analysis, single-gene approach, or whole-exome sequencing, approximately 25–40% of children with ISS are found to have an underlying specific genetic etiology.[3] Specific genes associated with ISS include defects/mutations of genes such as SHOX, aggrecan (ACAN), fibroblast growth factor receptor-3, natriuretic peptide receptor-2, or components of the growth hormone (GH)/insulin-like growth factor-1 (IGF-1) axis.[4] By definition, children with ISS are not GH deficient, but some may have subnormal levels of circulating IGF-1.[5] GH has been most commonly tried in the treatment of ISS and is recommended for use in children with ISS in some countries across the globe. It must be realized that heterogeneity in the underlying pathology explains the variable response to GH therapy in children with ISS.[5]
In a meta-analysis, we recently reported that letrozole is safe and effective for improving height and pubertal outcomes in CDGP and is better than testosterone with regard to improvement in testicular volume and delayed bone age (BA) progression.[6] First-generation aromatase inhibitors (AIs) like testolactone are no longer available for clinical use since 2008.[7] This is due to weak inhibitory activity and moderate clinical response, which prevented its widespread use.[7] Third-generation AIs such as letrozole or anastrozole effectively suppress estrogen biosynthesis and have also been shown to delay bone maturation in children with ISS, resulting in an increased predicted adult height (PAH) following 1–3 years of treatment.[8] There are several studies which have evaluated the role of letrozole in ISS.[8] Concerns have been raised with regard to the inhibition of estrogen biosynthesis in pubertal children and the potential impact on bone health and spinal deformities. In a recently published network meta-analysis of four studies, Wang et al. noted that both AIs and gonadotropin-releasing hormone agonist monotherapy were effective in augmenting the final height of boys with ISS, as compared to placebo.[9] However, the limitation of this network meta-analysis is that they missed out on several RCTs which evaluated the role of AIs in ISS.[9] Also, instead of four, they actually analyzed data from three studies, as Hero et al. and Tero represent two papers from the same cohort of patients.[9] Liu et al. published a systematic review and meta-analysis in 2021 highlighting that the use of AIs is associated with improvement in final height and PAH in children with short stature.[10] However, they analyzed children with CDGP and ISS together.[10] Children with CDGP and ISS have totally different growth trajectories. This makes it difficult for us to take the results into perspective. Furthermore, they analyzed data from studies using testolactone, which is no longer used. Hence, there remained a need for a dedicated meta-analysis to holistically analyze and summarize the clinical efficacy and safety of AIs used only in ISS. Hence, this meta-analysis aimed to evaluate the efficacy and safety of third-generation AIs in the management of ISS.
MATERIAL AND METHODS
The meta-analysis was carried out according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions.[11] The predefined protocol has been registered in PROSPERO, having Registration Number of CRD42021250557. All randomized controlled trials (RCTs) published till March 2023 were considered for this meta-analysis. This meta-analysis has been reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.[11] Since ethical approval already exists for the individual studies included in the meta-analysis, no separate approval was required for this study.
The PICOS criteria were used to screen and select the studies for this meta-analysis. Only children with ISS were considered for this meta-analysis. Children with other forms or causes of short stature, such as CDGP, FSS, GH deficiency (GHD)-induced short stature, panhypopituitarism/multiple pituitary hormone deficiency, and syndromes causing short stature were excluded from the study.
The primary outcome was to evaluate changes in PAH. Secondary outcomes were to evaluate changes in height standard deviation score (Ht-SDS), BA, testicular volume, hormonal markers of puberty, bone mineral density (BMD), and any side effects reported. Analysis of the growth and puberty outcomes was done based on whether the control group received an active comparator (such as GH, androgens, or any other approved medication for use in ISS) – marked as active control group (ACG) or placebo – marked as placebo/passive control group (PCG).
A detailed electronic database of Embase (through Ovid SP), Medline (through PubMed), ctri. nic.in, Cochrane Central Register of Controlled Trials (CENTRAL) (for trials only), clinicaltrials.gov, Google Scholar, and global health were searched using Boolean search strategy: [(aromatase inhibitors) OR (letrozole) OR (anastrozole)] AND [(short stature) OR (idiopathic short stature)].
Data extraction was carried out independently by two authors using standard data extraction forms. Details have been elaborated in a similar meta-analysis done in children with CDGP elsewhere.[6] Three authors independently assessed the risk of bias using the risk of bias assessment tool in Review Manager (RevMan) Version 5.4 (The Cochrane Collaboration, Oxford, UK 2014) software. The different types of bias looked for have already been elaborated on previously.[6]
For continuous variables, outcomes were expressed as mean differences (MD). SI units were used for analysis. For dichotomous outcomes (treatment success), results were expressed as risk ratios (RR) with 95% confidence intervals (CI). RevMan 5.4 was used for comparing MD of the different outcomes between AIs and controls. Heterogeneity was initially assessed by studying the forest plot generated for the primary and secondary outcomes of this study. Subsequently, heterogeneity was analyzed using a Chi-square test on N-1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I2 test.[12] Details of the interpretation of heterogeneity have been elaborated elsewhere.[6].
An overall grading of the evidence (certainty of the evidence) related to each of the primary and secondary outcomes of the meta-analysis was done using the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) approach.[13] The details of how the GRADEpro Guideline Development Tool software (McMaster University and Evidence Prime Inc, 2015) was used to create the Summary of Findings table have been elaborated elsewhere.[6] Publication bias was assessed by plotting the Funnel Plots.[10] The presence of one or more of the smaller studies outside the inverted funnel plot was taken as evidence of the presence of significant publication bias.[14]
A random effect model was used for analysis in this meta-analysis using RevMan 5.4 software. P < 0.05 was considered statistically significant.
RESULTS
A total of 1380 articles were found after the initial search [Figure 1]. Sixty-eight duplicates were removed. Following the screening of the titles and abstracts, the search came down to 158 articles. Ninety articles were reviewed in detail from which four RCTs which fulfilled all inclusion and exclusion criteria were included in the meta-analysis [Figure 1].[15-18] Hero et al. published three research papers from their cohort of patients in 2005,[15] in 2009,[19] and in 2019.[8] The paper by Hero et al. primarily looked into the cognitive outcomes of children receiving letrozole as compared to placebo,[19] whereas the paper published in 2005 looked into the growth outcomes.[15] The paper by Varimo et al.[8] is an extension of the paper by Hero et al.[15] Hence, their data have been merged for analysis and presented under Hero et al. Hence, the results of the three papers of Hero et al. have been analyzed together as they represent the same set of patients.[8,15,19] The RCT by Salehpour et al. was excluded as it was done in children with CDGP and not ISS.[20] Three studies were not included in the meta-analysis as they were not RCTs.[21-23]
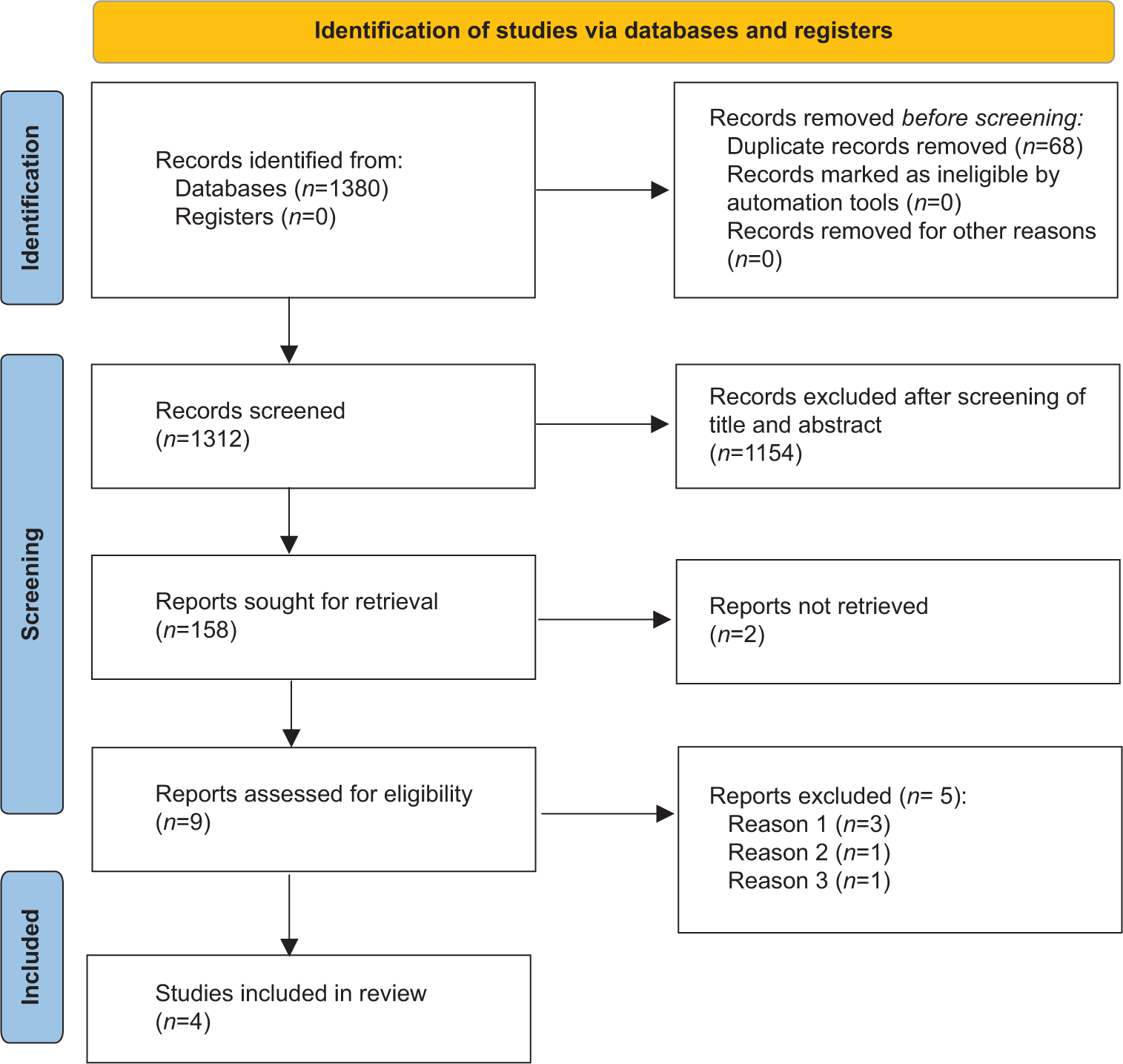
- Flowchart elaborating on study retrieval and inclusion in the meta-analysis.
Letrozole was the AI used in the studies by Hero et al.[15] The AI was anastrozole in the study by Rothenbuhler et al.[17] Both anastrozole or letrozole was used in the study by Mauras et al.[16] and Kong et al.[18] Of the four studies included in this meta-analysis, analysis was done based on the nature of the control group. The study by Mauras et al. had three groups comparing outcomes of children with ISS receiving AIs (letrozole/anastrozole), GH, and AIs (letrozole/anastrozole) with GH.[16] The outcomes of children receiving AIs as compared to those receiving GH from this study were included in this meta-analysis under the ACG.[16] The quality-of-life outcomes of children receiving AIs and GH in the study by Bullinger et al. were published as a separate research paper.[24] It has been discussed in detail later. However, since it involved the same cohort of children, its results have not been analyzed separately with regard to the primary and secondary outcomes of this meta-analysis. The 3 reasons for the exclusion of certain studies from analysis in Figure 1 have been elaborated here. Reason 1: 3 studies were excluded as they were not RCTs; Reason 2: Hero et al.[15,19] had published two papers from the same cohort of patients; hence, they were combined for analysis. Reason 3: The study by Salehpour et al.[20] was done in patients with constitutional delay in growth and puberty and not idiopathic short stature as was wrongly apparent from the title.
The controls were placebo in the studies by Hero et al.,[15] Rothenbuhler et al.,[17] and Kong et al.,[18] and hence, the results from these studies have been analyzed in the PCG. However, it must be remembered that both the study and the control group received GH in the studies by Rothenbuhler et al. and Kong et al.[17,18] The details of the studies included in this meta-analysis are shown in Table 1. The study by Li et al. was excluded from the analysis as the study group children received AIs with spironolactone. In the presence of spironolactone only in the study group, it would not be easy to take the results into perspective due to the anti-androgenic properties of spironolactone.[23]
| Study details | Number of patients on aromatase inhibitors and control groups | Patient characteristics and nature of controls | Duration of study |
|---|---|---|---|
| Hero et al. 2005[15] | Boys with ISS were treated with the AI letrozole (2.5 mg/day) or placebo | Thirty-one boys aged 9.0–14.5 years with ISS were studied | Two-year follow-up duration |
| Mauras et al. 2016[16] | Seventy-six pubertal boys having mean (SE) age of 14.1 (0.1) years and height SDS score of −2.3 (0.0) | This study compared the efficacy and safety of AIs versus GH versus AIs along with GH on increasing adult height potential in pubertal boys with severe ISS. | Two-year follow-up duration |
| Rothenbuhler et al. 2016[17] | 24 healthy adolescent boys; anastrozole was the AI | A prospective randomized study comparing GH with AIs versus GH alone was conducted in adolescent boys aged 15.2±1.2 years with serum testosterone at adult levels and a faltering growth velocity <3.5 cm/year leading to PAH <2.5 SDS. Treatments were stopped when growth velocity became <10 mm in 6 months or when height was close to 170 cm. | 2–3 years of follow-up duration |
| Kong et al. 2020[18] | rhGH+AI group (n=108) and rhGH group (n=43) | A total of 151 short-stature pubertal boys with ages of 10–14 years and bone age of 13–15 years were studied. All children were injected subcutaneously with rhGH 0.15-0.2 IU/kg/day, and those in the rhGH+AI group were additionally given 2.5 mg/d letrozole or one mg/d anastrozole | 20 months to 2 years follow-up duration |
AI: Aromatase inhibitor, ISS: Idiopathic short stature, SDS: Standard deviation score, GH: Growth hormone, PAH: Predicted adult height, rhGH: Recombinant human growth hormone, SE: Standard error
Risk of bias in the included studies
The summaries of the risk of bias of the four studies included in the meta-analysis are shown in Figures 2a and b. Random sequence generation was low risk in three out of four studies (75%). Reporting bias and attrition bias were judged to be at low risk of bias in all four studies (100%). Source of funding, especially pharmaceutical, authors from the pharmaceutical organizations, and conflict of interests were looked into the “other bias” section. Other bias was low risk in three out of four studies (75%). Allocation concealment bias (selection bias) was low risk in two out of four studies (50%). Performance bias (blinding of participants and investigators) and detection bias (blinding of outcome assessors) were judged to be at low risk in one out of four studies (25%).
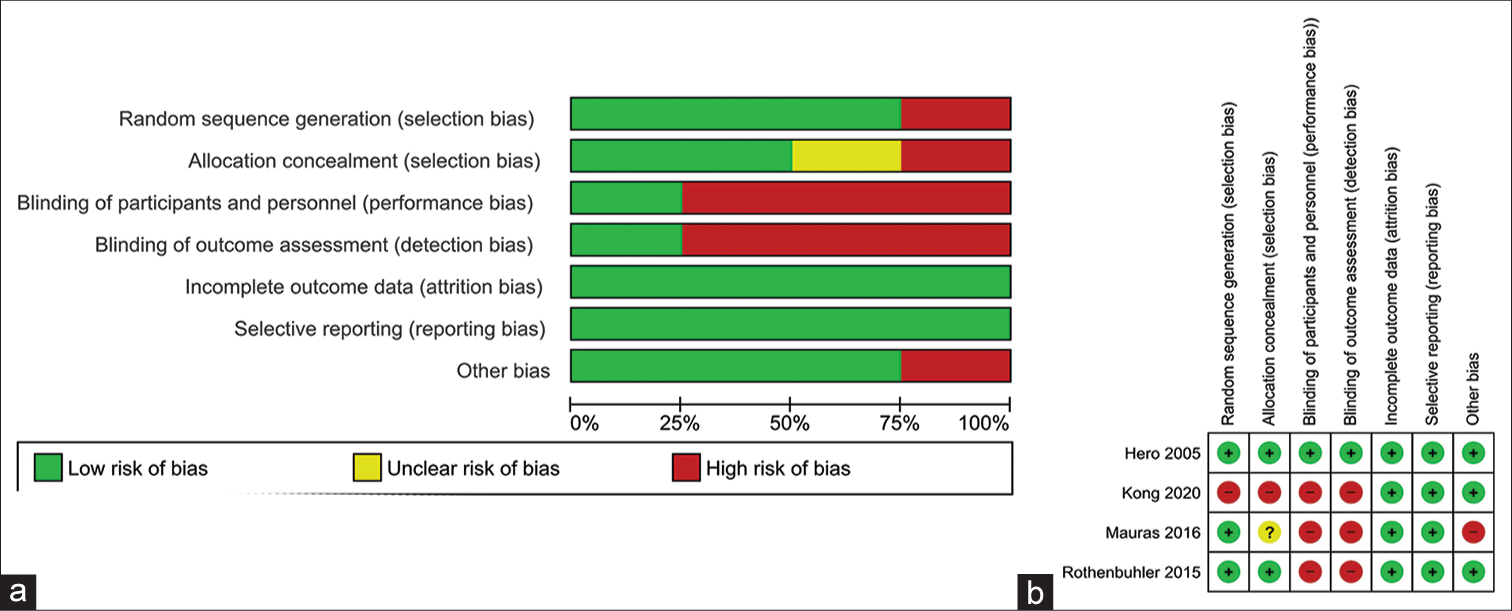
- (a) Risk of bias graph: Review authors’ judgments about each risk of bias item presented as percentages across all included studies; (b) risk of bias summary: Review authors’ judgments about each risk of bias item for each included study.
Effect of AIs on primary outcomes
PAH
In the studies by Mauras et al., [16] Hero et al.,[15] and Kong et al.,[18] PAH was calculated using Bayley Pinneau tables.[25] In the study by Rothenbuhler et al.,[17] the authors did not use the Bayley Pinneau method for calculating PAH.[25] Instead, the authors modeled the deceleration of growth velocity in each adolescent to be able to extrapolate adult height, using the age and height of each child measured during the deceleration periods. The authors considered that growth velocity thereafter slows uniformly and is terminated within two years from the peak growth velocity.[17]
Data from one study involving 50 children with ISS were analyzed to find out the impact of AIs on the changes in PAH after 24 months of treatment when compared to those receiving GH in the control group (ACG). Individuals receiving AIs had significantly lower improvement in height outcomes as compared to those receiving GH in the ACG (MD −2.50 cm [95% CI: −3.31–−1.69]; P < 0.01; [Figure 3a]). Data from three studies involving 192 children with ISS were analyzed to find out the impact of AIs on PAH after at least 12 months of treatment when compared to those receiving a placebo (PCG). Individuals receiving AIs had a significantly greater improvement in PAH when compared to PCG (MD 4.62 cm (95% CI: 4.02–5.23); P < 0.01; I2 = 0% [low heterogeneity]; [Figure 3b]).
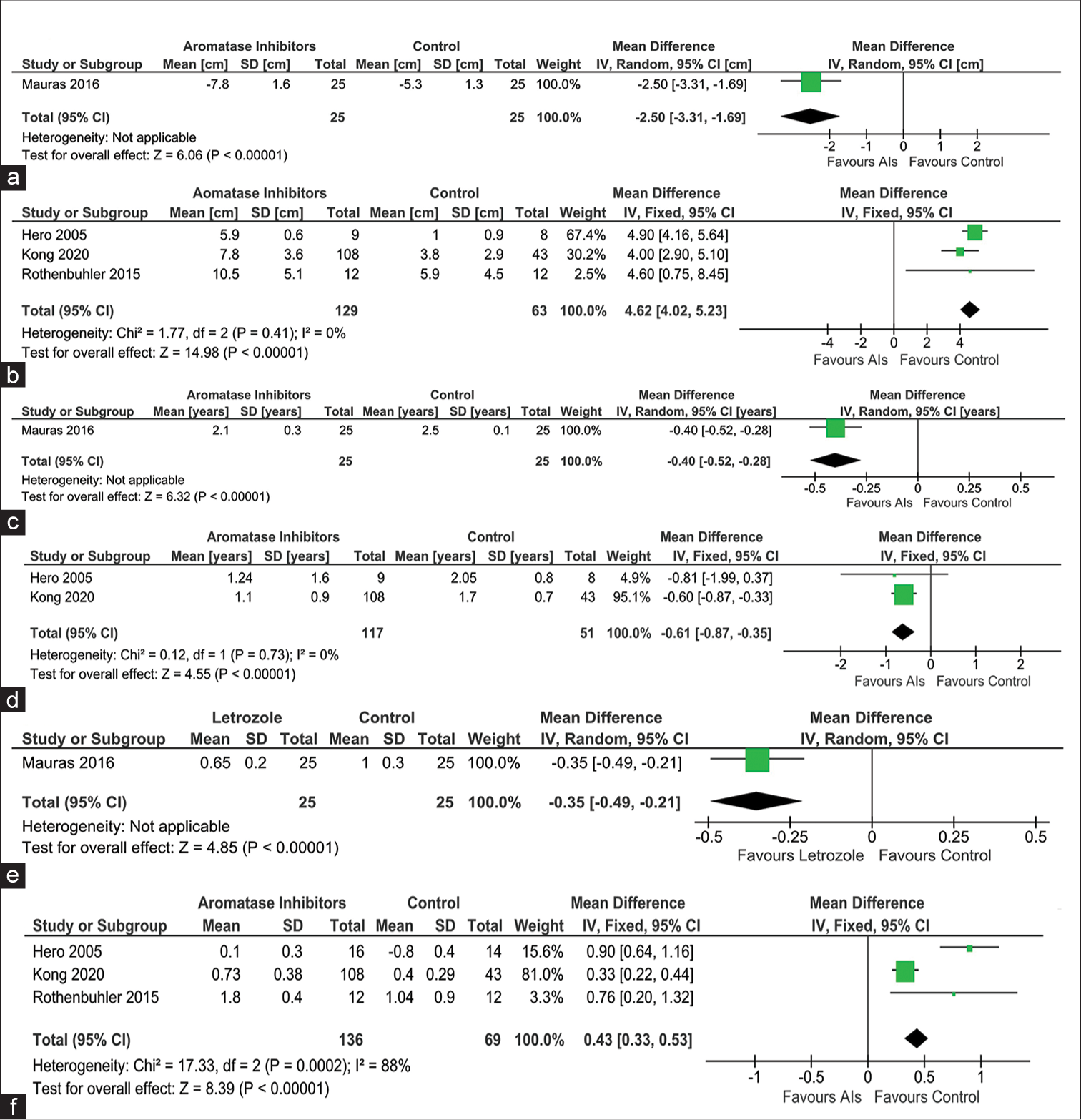
- Forest plot highlighting the impact of aromatase inhibitors (AIs) on (a) predicted adult height (PAH) as compared to active controls receiving growth hormone (GH); (b) PAH as compared to passive controls receiving placebo; (c) bone age (BA) progression as compared to active controls receiving GH; (d) BA progression as compared to passive controls receiving placebo; (e): height standard deviation score (Ht-SDS) changes as compared to active controls receiving GH; and (f): height standard deviation score (Ht-SDS) changes as compared to passive controls receiving placebo, AI: Aromatase inhibitor, CI: Confidence interval, SD: Standard deviation, IV: Instrumental variable.
Effect of AIs on secondary outcomes
BA
Data from one study involving 50 children were analyzed comparing the changes in BA after 24 months of therapy with AIs, as compared to those receiving GH in the ACG. When compared to ACG, children receiving AIs had a significantly slower progression in BA (MD −0.40 years [95% CI: −0.52–−0.28]; P < 0.01; [Figure 3c]). Data from two studies involving 168 children were analyzed comparing the changes in BA after at least 12 months of therapy with AIs, as compared to those receiving placebo (PCG). When compared to PCG, children receiving AIs had a significantly slower progression in BA (MD −0.61 years [95% CI: −0.87–−0.35]; P < 0.01; I2 = 0% [low heterogeneity]; [Figure 3d]).
Ht-SDS
Data from one study involving 50 children were available comparing the changes in Ht-SDS after at least 24 months of therapy in ACG. Children receiving AIs had a significantly lesser improvement in Ht-SDS when compared to those receiving GH in ACG (MD −0.35 [95% CI: −0.49–−0.21]; P < 0.01; [Figure 3e]). Data from three studies involving 74 children were analyzed comparing the changes in Ht-SDS after at least 12 months of therapy with AIs, as compared to those receiving placebo (PCG). When compared to PCG, children receiving AIs had a significantly greater improvement in Ht-SDS (MD 0.43 [95% CI: 0.33–0.53]; P < 0.01; I2=88% [moderate heterogeneity]; [Figure 3f]).
Testicular volume
Data from one study involving 50 children were available comparing the changes in testicular volume after at least 24 months of therapy in ACG. Children receiving AIs had greater increase in testicular volume when compared to those in ACG (MD 5.00 mL [95% CI: 2.34–7.66]; P < 0.01; [Figure 4a]. Data from one study involving 17 children with ISS were analyzed to find out the impact of AIs on testicular volume after at least 12 months of treatment when compared to those receiving a placebo (PCG). Children receiving AIs had a greater increase in testicular volume when compared to those in PCG (MD 6.50 mL [95% CI: 3.69–9.31]; P = 0.30; [Figure 4b], which was not statistically significant.
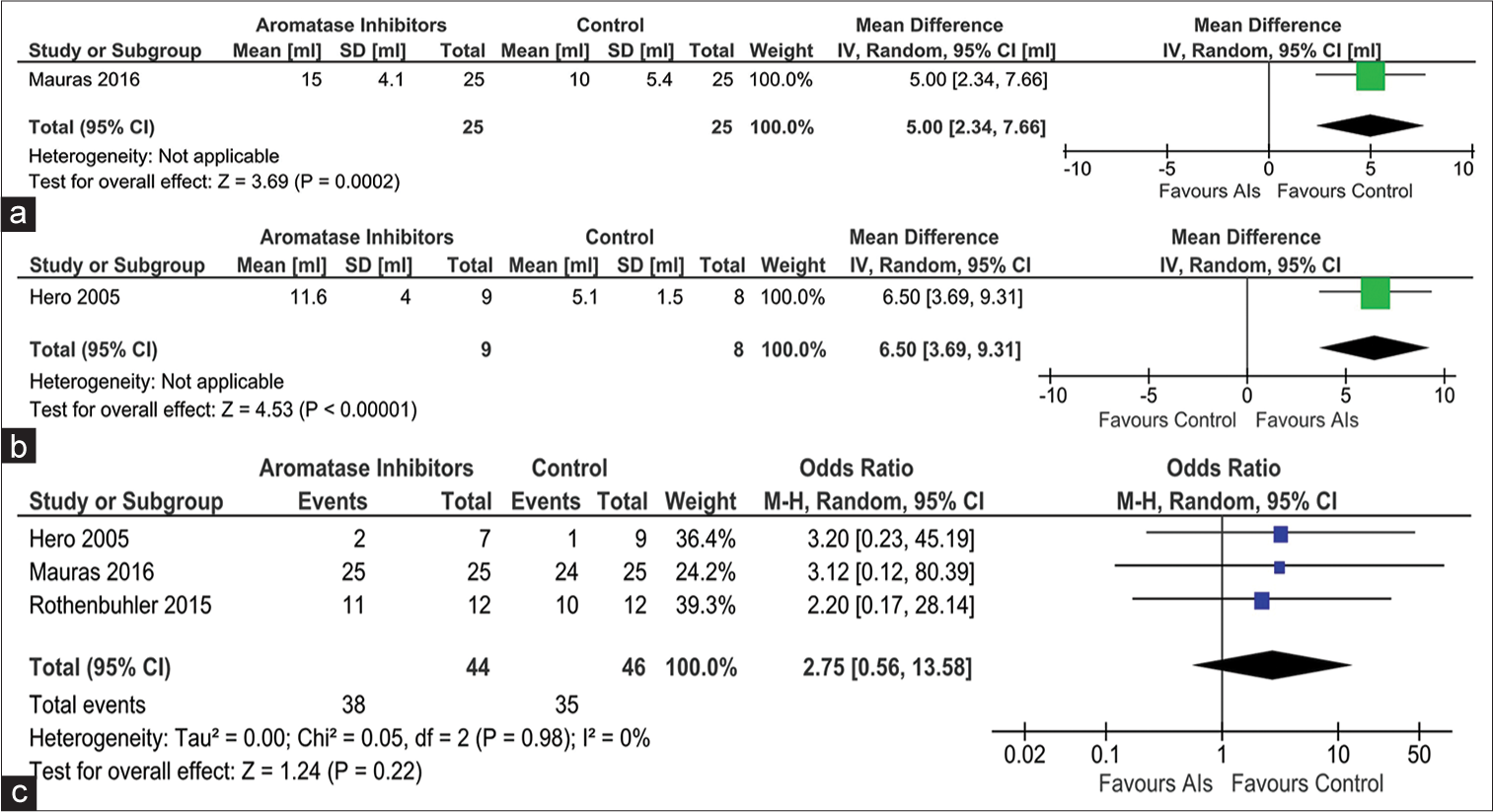
- Forest plot highlighting the impact of aromatase inhibitors on (a) progression in testicular volume as compared to active controls receiving growth hormone; (b) progression in testicular volume as compared to passive controls receiving placebo; and (c) occurrence of total adverse events, AI: Aromatase inhibitor, CI: Confidence interval, SD: Standard deviation, IV: Instrumental variable.
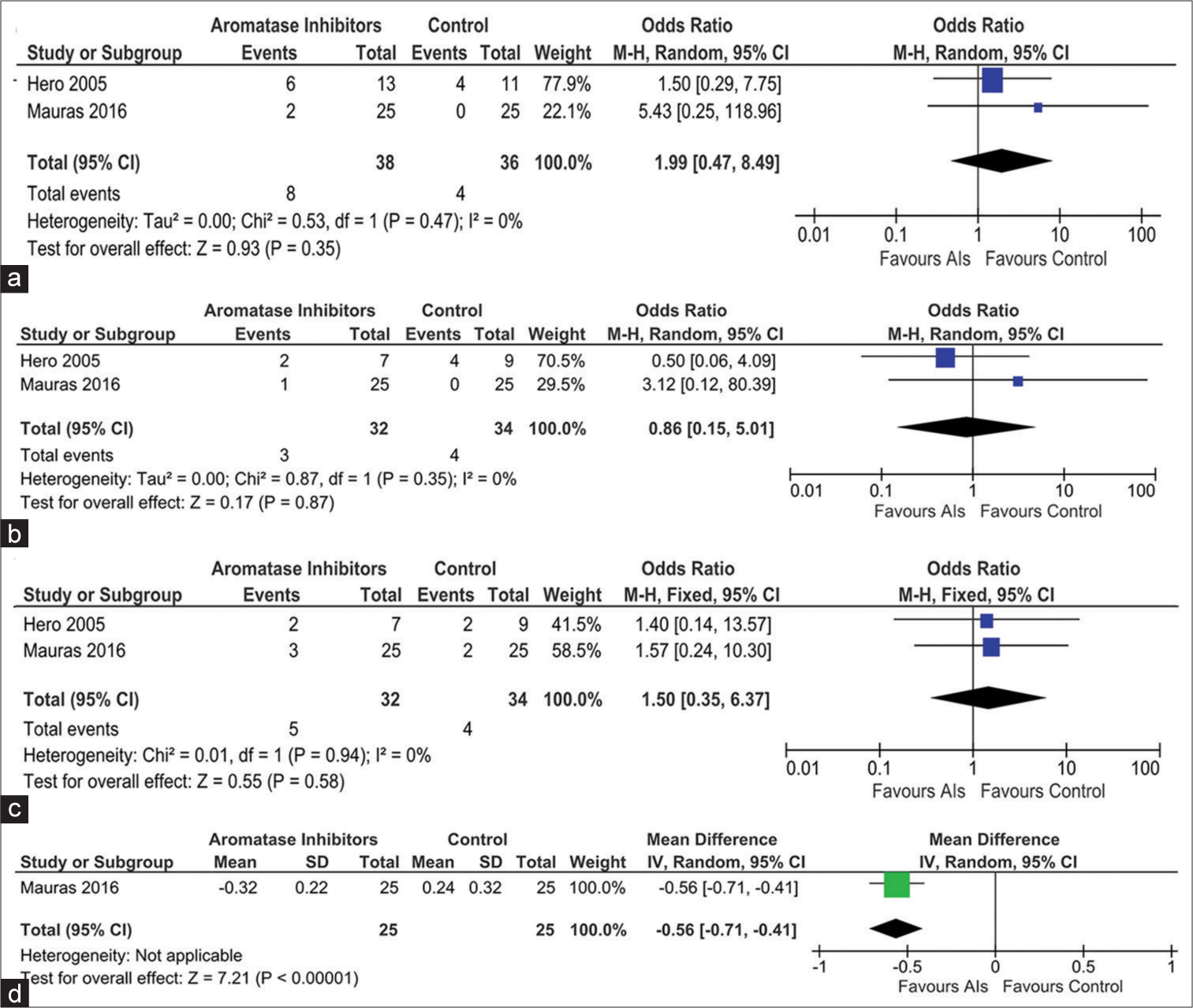
- Forest plot highlighting the side effect profile of the use of aromatase inhibitors in idiopathic short stature focusing on (a) vertebral deformity, (b) end-plate deformity, (c) intervertebral disc abnormality, and (d) lumbar spine bone mineral density, AI: Aromatase inhibitor, CI: Confidence interval, SD: Standard deviation, IV: Instrumental variable, M-H: Mantel–Haenszel.
Testosterone
Data from one study involving 50 children (Mauras et al.) were available, comparing the changes in serum testosterone after at least 24 months of therapy in ACG.[16] Children receiving AIs had a significantly higher increase in serum testosterone when compared to those in ACG receiving GH (MD 20.27 nmol/L [95% CI: 19.49–21.05]; P < 0.01). Data from one study involving 151 children (Kong et al.) were available, comparing the changes in serum testosterone after around 24 months of therapy in PCG.[16,18] Children receiving AIs had a significantly higher increase in serum testosterone when compared to those in PCG (MD 18.10 ng/dL [95% CI: 17.17–19.03]; P <0.01). In this study, both the study and the control groups received GH.
Safety
Data from three studies (90 children) were analyzed to evaluate the impact of AIs on the occurrence of total adverse events (TAEs) over 1–2 years of treatment. The occurrence of TAEs (Risk ratio [RR] 2.75 [95% CI: 0.56–13.58]; P = 0.22; I2 = 0% [low heterogeneity]; [Figure 4c] was not statistically different in children receiving AIs as compared to controls. The occurrence of vertebral deformity was evaluated in the studies by Hero et al. and Mauras et al.[15,16] The occurrence of vertebral deformity (RR 1.99 [95% CI: 0.47–8.49]; P = 0.35; I2 = 0% [low heterogeneity]; [Figure 5a], end-plate deformity (RR 0.86 [95% CI: 0.15–5.01); P = 0.87; I2 = 0% [low heterogeneity]; [Figure 5b], and inter-vertebral disc abnormality (RR 1.50 [95% CI: 0.35–6.37]; P = 0.58; I2 = 0% [low heterogeneity]; [Figure 5c]) were not statistically different in children receiving AIs as compared to controls.[15,16]
Bone mineral density
Data from one study involving 50 children (Mauras et al.) were available, comparing the changes in BMD Z-scores at the lumbar spine after at least 24 months of therapy in ACG.[16] Children receiving AIs had a greater decline in BMD Z-scores when compared to those in ACG receiving GH (MD −0.56 kg/m2 [95% CI: −0.71–−0.41]; P < 0.01); [Figure 5d]. In the study by Kong et al., five children (on AI with GH therapy) had lower limb fractures; one child due to jumping from a windowsill and causing a tibial fracture; one child with accidental fall leg fracture; and three children with sports injury-related fracture.[18] All fractures were associated with significant trauma and likely unrelated to the study medications.[18]
The key summary of the findings of this meta-analysis is shown in Table 2. Funnel plots plotted to evaluate the presence of publication bias are shown in Supplementary Figure 1.
| Intervention: AIs Comparison: Control |
|||||
|---|---|---|---|---|---|
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with Control | Risk with AIs | ||||
| PAH PCG | The mean PAH PCG was 161.2cm | MD 4.94 cm higher(4.24 higher to 5.65 higher) | - | 61 (3 RCTs) | ⨁⨁⨁⨁HIGH |
| Bone age PCG | The mean bone age PCG was 9.95years | MD 0.93 years lower(1.84 lower to 0.03 lower) | - | 37 (2 RCTs) | ⨁⨁⨁⨁HIGH |
| Ht-SDS PCG | The mean Ht-SDS PCG was -2.26 | MD 0.77 higher(0.55 higher to 0.98 higher) | - | 74 (3 RCTs) | ⨁⨁◯◯ LOWa,b |
| TAEs | 761/1,000 | 897/1,000(641–977) | OR 2.75(0.56–13.58) | 90 (3 RCTs) | ⨁⨁⨁⨁HIGH |
| Vertebral deformity | 111/1,000 | 217/1,000(72–498) | OR 2.22(0.62–7.93) | 90 (3 RCTs) | ⨁⨁⨁⨁HIGH |
| End-plate deformity | 118/1,000 | 103/1,000(20–400) | OR 0.86(0.15–5.01) | 66 (2 RCTs) | ⨁⨁⨁⨁HIGH |
| Intervertebral disc abnormality | 118/1,000 | 167/1,000(45–459) | OR 1.50(0.35–6.37) | 66 (2 RCTs) | ⨁⨁⨁⨁HIGH |
GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect
DISCUSSION
A previous Cochrane meta-analysis published by McGrath and O’Grady noted the beneficial impact of AIs to improve short-term growth outcomes like PAH in male children and adolescents with short stature.[26] However, the limitation of that meta-analysis was that they included children with heterogenous etiologies of short stature, namely, CDGP, ISS, as well as GHD, making it difficult to take the results into perspective when focusing on ISS.[26]
An important observation from this meta-analysis is AIs use in children with ISS, which is associated with a significantly greater improvement in PAH when compared to placebo. Our analysis showed that the use of AIs in children with ISS resulted in a mean improvement in PAH +4.94 cm. This improvement in PAH with AIs was associated with a significantly slower progression in BA and a significantly better improvement in testicular volume and height SDS scores as compared to placebo. Hence, AIs have a role in improving both the height outcomes and the pubertal outcomes in children with ISS.
AIs were noted to be inferior to GH with regard to height improvement (improvement in PAH and Ht-SDS scores) in children with ISS. This is very much expected. An important observation of this meta-analysis is that AIs were superior to GH with regard to improving pubertal outcomes in children with ISS, namely, slowing of BA progression along with improvement in testicular volume and serum testosterone levels. Hence, there is a scope of using AIs and GH together in children with ISS. AIs would delay the BA progression and, hence, help in improving the height outcomes with a longer duration of GH therapy.
It is important to note that no RCT data were available that compared the outcomes of AIs versus Low-dose testosterone in children with ISS; hence, such an analysis could not be done in children with ISS. Similar comparisons are, however, available in children with CDGP.[6]
This meta-analysis provided assuring data regarding the long-term safety of AIs use in ISS. No increased occurrence of TAEs and severe adverse events were noted with the use of AIs. The occurrence of vertebral deformity, end-plate deformity, and inter-vertebral disc abnormalities was not increased with the use of AIs as compared to controls. Mauras et al. noted that there was a significant decline in lumbar spine BMD Z-scores over two years of use of AIs as compared to controls receiving GH. GH is well known for its anabolic effects on bone, resulting in an increase in BMD.[16] This increase would have accentuated the observation of a decline in BMD Z-scores at the lumbar spine with AIs. Hence, the true impact of AIs in BMD cannot be established from the observations by Mauras et al.[16] AIs, by virtue of their mechanism of action, are associated with lower estrogen levels, which explains the slower progression of BA with their use. However, the trophic effect of estrogen on BMD may be attenuated with the use of AIs in children. Hence, we need dedicated studies evaluating BMD outcomes in children receiving AIs as compared to placebo to establish long-term BMD outcomes.
Limitations of this meta-analysis include the small number of available RCTs which were analyzed. For many of the outcomes, only one or two studies were available. Furthermore, significant data heterogeneity with regard to some of the outcomes reduces the quality of the data generated. Furthermore, we must note that the information on the final height of these children was not available from the individual studies. Hence, we could not analyze the same and it is a limitation of this meta-analysis. Hence, there is an urgent need for more RCTs with a larger number of children with a longer duration of follow-up evaluating the role of AIs in ISS.
CONCLUSION
It may be said that our meta-analysis on the efficacy and safety of AIs in ISS provides us with reassuring data on the good efficacy and tolerability of this class of medication on height outcomes and pubertal progression outcomes in ISS. There remains scope for using AIs and GH together in children with ISS to have a synergistic impact on height outcomes. Dedicated long-term studies are needed in this area.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient consent is not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Supplementary file available on:
Financial support and sponsorship
Nil.
References
- Consensus statement on the diagnosis and treatment of children with idiopathic short stature: A summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. J Clin Endocrinol Metab. 2008;93:4210-7.
- [CrossRef] [PubMed] [Google Scholar]
- The challenge of defining and investigating the causes of idiopathic short stature and finding an effective therapy. Horm Res Paediatr. 2019;92:71-83.
- [CrossRef] [PubMed] [Google Scholar]
- A genetic approach to evaluation of short stature of undetermined cause. Lancet Diabetes Endocrinol. 2018;6:564-74.
- [CrossRef] [PubMed] [Google Scholar]
- Next generation sequencing-based mutation screening of 86 patients with idiopathic short stature. Endocr J. 2017;64:947-54.
- [CrossRef] [PubMed] [Google Scholar]
- Standard and low-dose IGF-I generation tests and spontaneous growth hormone secretion in children with idiopathic short stature. Clin Endocrinol (Oxf). 2004;60:163-8.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of letrozole in the management of constitutional delay in growth and puberty: A systematic review and meta-analysis. J Clin Res Pediatr Endocrinol. 2022;14:131-44.
- [CrossRef] [PubMed] [Google Scholar]
- Testolactone: The rise and fall of a drug. Drugs Drug Candidates. 2023;2:69-94.
- [CrossRef] [Google Scholar]
- Letrozole monotherapy in pre-and early-pubertal boys does not increase adult height. Front Endocrinol (Lausanne). 2019;10:201.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative efficacy of aromatase inhibitors and gonadotropin-releasing hormone analogue in increasing final height of idiopathic short stature boys: A network meta-analysis. Front Endocrinol (Lausanne). 2023;14:1167351.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of short stature with aromatase inhibitors: A systematic review and meta-analysis. Horm Metab Res. 2021;53:391-401.
- [CrossRef] [PubMed] [Google Scholar]
- The PRISMA statement for reporting systematic reviews and metaanalyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339:b2700.
- [CrossRef] [PubMed] [Google Scholar]
- GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924-6.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of gemigliptin, a dipeptidyl peptidase-4 inhibitor, in patients with type 2 diabetes mellitus inadequately controlled with combination treatment of metformin and sulphonylurea: A 24-week, multicentre, randomized, double-blind, placebo-controlled study (TROICA study) Diabetes Obes Metab. 2017;19:635-43.
- [CrossRef] [PubMed] [Google Scholar]
- Inhibition of estrogen biosynthesis with a potent aromatase inhibitor increases predicted adult height in boys with idiopathic short stature: A randomized controlled trial. J Clin Endocrinol Metab. 2005;90:6396-402.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized trial of aromatase inhibitors, growth hormone, or combination in pubertal boys with idiopathic, short stature. J Clin Endocrinol Metab. 2016;101:4984-93.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized pilot trial of growth hormone with anastrozole versus growth hormone alone, starting at the very end of puberty in adolescents with idiopathic short stature. Int J Pediatr Endocrinol. 2015;2015:4.
- [CrossRef] [PubMed] [Google Scholar]
- Aromatase inhibitors combined with growth hormone in treatment of adolescent boys with short stature. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49:283-90. [Chinese]
- [Google Scholar]
- Impact of aromatase inhibitor therapy on bone turnover, cortical bone growth and vertebral morphology in pre-and peripubertal boys with idiopathic short stature. Horm Res. 2009;71:290-7.
- [CrossRef] [PubMed] [Google Scholar]
- A double-blind, placebo-controlled comparison of letrozole to oxandrolone effects upon growth and puberty of children with constitutional delay of puberty and idiopathic short stature. Horm Res Paediatr. 2010;74:428-35.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical effect of letrozole in treatment of idiopathic short stature in adolescent boys. Zhongguo Dang Dai Er Ke Za Zhi. 2019;21:977-82. [Chinese]
- [Google Scholar]
- Height outcomes in children with growth hormone deficiency and idiopathic short stature treated concomitantly with growth hormone and aromatase inhibitor therapy: Data from the ANSWER program. Int J Pediatr Endocrinol. 2020;2020:19.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of letrozole in treatment of male adolescents with idiopathic short stature. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49:308-14. [Chinese]
- [Google Scholar]
- Quality of life in adolescent boys with idiopathic short stature: Positive impact of growth hormone and aromatase inhibitors. Horm Res Paediatr. 2018;90:381-92.
- [CrossRef] [PubMed] [Google Scholar]
- Tables for predicting adult height from skeletal age: Revised for use with the Greulich-Pyle hand standards. J Pediatr. 1952;40:423-41.
- [CrossRef] [PubMed] [Google Scholar]
- Aromatase inhibitors for short stature in male children and adolescents. Cochrane Database Syst Rev. 2015;2015:CD010888.
- [CrossRef] [Google Scholar]







