Translate this page into:
Acute insulin-induced hypoglycemia in twin neonates

*Corresponding author: J. Anita Chrisbina, Department of Neonatology, Mehta Multispeciality Hospital, Chennai, Tamil Nadu, India. j.anitachrisbina@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Chrisbina AJ, Krishnan BA, Prasad H. Acute insulin-induced hypoglycemia in twin neonates. J Pediatr Endocrinol Diabetes. 2023;3:114-7. doi: 10.25259/JPED_39_2023
Abstract
Twin neonates with a history of exogenous insulin administration by a mother suffering from post-partum psychosis are reported. Twin 1 presented with lethargy, while twin 2 presented with apnea and bradycardia, requiring vigorous resuscitation. During their neonatal intensive care unit stay, both the babies developed multiple episodes of symptomatic refractory hypoglycemia with seizures and were managed with intravenous dextrose boluses and infusion, glucagon infusion, octreotide, diazoxide, and glucocorticoids. Although short-acting regular insulin (Actrapid, Novo Nordisk™) preparation was administered to the neonates, its effects were seen for a duration of 3–4 days, probably due to the depot effect. We highlight the importance of history taking and physical examination for early diagnosis and appropriate management.
Keywords
Insulin toxicity
Neonatal hypoglycemia
Glucose infusion rate
Glucocorticoids
Glucagon
INTRODUCTION
Insulin poisoning in neonates is extremely rare. Here we report the challenges faced in managing twin preterm neonates with exogenous insulin overdose.
CASE REPORT
Preterm twin neonates of gestation 33 weeks + 1 day at birth, male and female with birth weight of 1.5 kg and 1.49 kg for twin 1 and 2, respectively, were delivered to a 38-year-old G5P2L0A2 mother, who conceived following in vitro fertilization (IVF) in view of recurrent abortions, with initial stay in a neonatal intensive care unit for two weeks for respiratory distress syndrome and preterm care, presented to the emergency room on day 30 of life with history of lethargy following exogenous insulin administration by the mother. The mother was on short-acting regular insulin (Actrapid, Novo Nordisk™) in view of gestational diabetes mellitus and was suffering from post-partum psychosis, and the father had witnessed her giving some injections to the babies. The mother did not have any history of psychiatric illness. Twin 1 was lethargic and irritable on admission with capillary blood glucose (BG) 40 mg/dL. The baby was hemodynamically stable and received an intravenous (IV) bolus of 10% dextrose followed by IV dextrose infusion with a glucose infusion rate (GIR) of 6 mg/kg/min.
Although the parents were advised to bring the second twin immediately, they brought the baby only on the following day with apnea and bradycardia with documented hypoglycemia requiring extensive resuscitation, including intubation. The baby was initiated on IV dextrose infusion with a GIR of 6 mg/kg/min after an IV bolus of 10% dextrose. Strict BG monitoring and baseline investigations were sent. Serum sodium was 131 mEq/L for twin 1 and 132 mEq/L for twin 2and was managed conservatively. They had normal serum potassium and phosphorus levels.
Both the babies had recurrent hypoglycemia, and GIR was progressively increased up to 17 mg/kg/min through a peripherally inserted central catheter (PICC) line. Both twins developed hypoglycemic seizures and received glucagon boluses followed by IV glucagon infusion of 20 µg/kg/h. The septic screen was negative for twin 1 and positive for twin 2, and antibiotics were initiated. Laboratory values of serum insulin were extremely high (more than 250 mIU/L) with negligible C-peptide levels (<0.1 ng/mL for twin 1 and 0.129 ng/mL for twin 2), confirming exogenous insulin administration. Octreotide infusion at 10 µg/kg/h, along with oral diazoxide, was initiated as a measure to correct persistent hypoglycemia. Hydrocortisone was also given at a dose of 10 mg/kg/day stat doses once or twice initially, but in view of persisting hypoglycemic episodes, glucocorticoids (parenteral hydrocortisone at 10 mg/kg/day initially followed by methylprednisolone 1–2 mg/kg/day) were also given. After 3–4 days, BG began to stabilize. Twin 2 was extubated after 48 hours of mechanical ventilation. Both were gradually weaned from octreotide and glucagon infusions, followed by tapering GIR. Feeds were initiated after the sensorium improved. Figures 1 and 2 show the trend of glucose levels and treatment in twin 1 and twin 2 respectively. Twin 1 was successfully discharged on the 8th day. Twin 2 developed post-extubation stridor, which gradually settled with conservative measures, and the baby was successfully discharged on day 14 of admission. These cases were reported to the police authorities. The mother was advised to consult the psychiatrist, and an appropriate referral was made to the psychiatrist for further care and management.
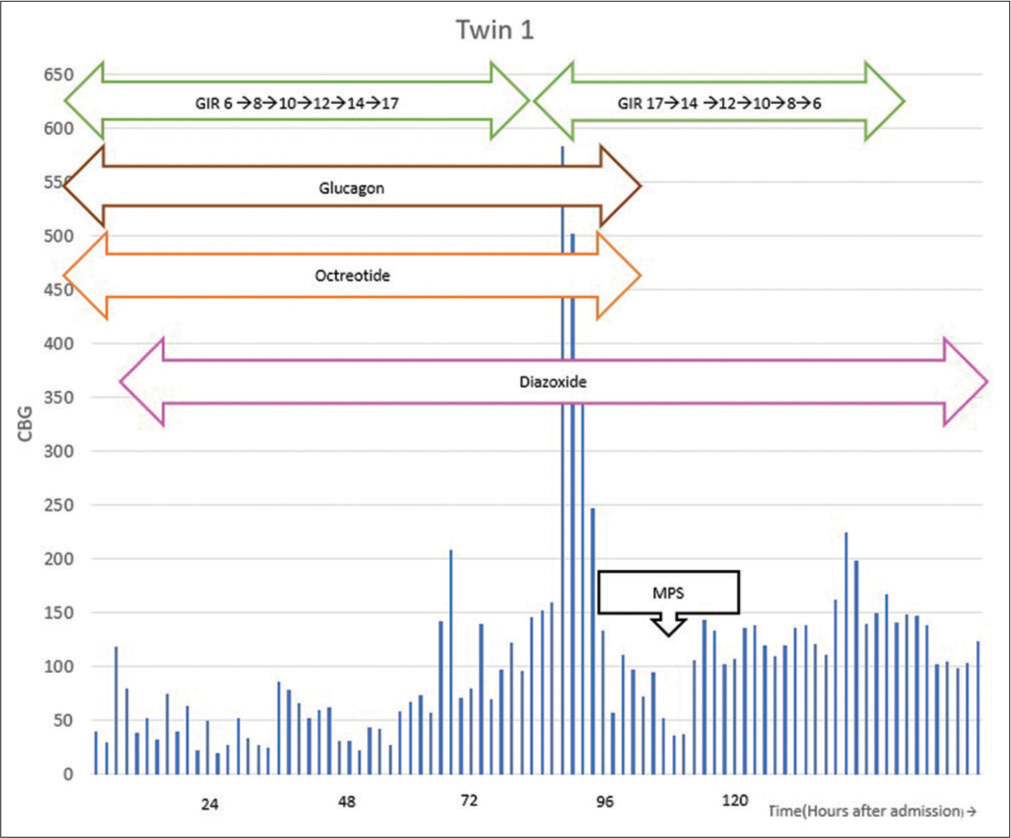
- Trend of glucose levels and treatment in twin 1. MPS: Methylprednisolone, GIR: Glucose infusion rate, CBG: Capillary blood glucose.

- Trend of glucose levels and treatment in twin 2. MPS: Methylprednisolone, GIR: Glucose infusion rate, CBG: Capillary blood glucose.
DISCUSSION
Insulin poisoning in neonates is extremely rare. The Pediatric Endocrine Society hypoglycemia guidelines published in 2015 state that BG level <50 mg/dL (plasma glucose <60 mg/dL) needs to be evaluated, and the threshold for treatment depends on the presence or absence of symptoms and associated risk factors.[1] In neonates, hypoglycemia has varied clinical presentations, such as irritability, tremor, jitteriness, lethargy, seizures, apnea, and poor feeding. Hypoglycemia in a growing preterm may be due to endogenous or exogenous hyperinsulinemia, in addition to endogenous causes such as drugs, tumors, and hormonal imbalance.
The exogenous nature of hyperinsulinemia was confirmed as the serum insulin levels were very high with negligible C-peptide levels. The mother was on short-acting regular insulin (Actrapid, Novo Nordisk™) in view of gestational diabetes and was suffering from postpartum psychosis, and the father had witnessed her giving some injections to the babies. This history was extremely useful in managing the twins appropriately. Although delayed effects are more common with long-acting insulins, short-acting regular insulin can also produce delayed effects. This is explained on the basis of the depot effect, even though the half-life of Actrapid is only 2–5 hours. Significant reduction in local blood flow occurs by compression of tissue at the injection site, especially when a large quantity of insulin is injected.
High doses of insulin can lead to dyselectrolytemia as well. Insulin excess leads to salt and water retention, causing dilutional hyponatremia. There can be an intracellular shift of potassium and phosphorus, leading to hypokalemia and hypophosphatemia.[2,3] Our patients had mild hyponatremia initially and were managed conservatively, along with normal potassium and phosphorus levels. Acute pulmonary edema can occur due to sympathetic activation.
The cornerstone of the management of hypoglycemia is IV dextrose.[2] As the plasma insulin levels increase and reach 50–60 µU/mL, the hepatic glucose output is suppressed completely, and glucose needs to be given exogenously. Whenever an episode of hypoglycemia occurred, 2 mL/kg of 10% dextrose (0.2 g/kg) IV boluses were given, followed by continuous IV dextrose infusion at a rate of 5–8 mg/kg/min. Plasma glucose levels should be obtained 20–30 min after initiation of IV therapy, and any increase in infusion rates and the dextrose concentration should be adjusted accordingly. The maximum rate of infusion is limited by the maximum amount of fluids that can be administered and the maximum concentration of dextrose for the type of vascular access. The maximum concentration of dextrose through a peripheral IV catheter is 12.5%, and the central catheter is 25%. In our cases, PICC lines were placed on the day of admission, and IV fluid preparations with 25% dextrose were used.
Glucose administration to neonates for up to 2 years should not exceed 17.2 g/kg/day or 12 mg/kg/min since this is the maximum rate of glucose oxidation.[4] Anecdotal case reports of using GIR up to 30 mg/kg/min have been reported, although hepatic injury may occur as hepatocytes are overloaded with the influx of glucose.[5] Thus, when GIR is more than 12 mg/kg/min, additional drugs need to be added. In our case, GIR was increased up to 17 mg/kg/min to achieve euglycemic status along with simultaneous initiation of second-line drugs. In our patients, the second-line drugs were initiated even earlier, anticipating refractory hypoglycemia.
Glucagon administration should be considered in patients with hypoglycemia despite maximum IV dextrose infusion. Glucagon helps to regulate gluconeogenesis and glycogenolysis within the liver as a counter-regulatory agent to insulin.[5-8] We suggest initiating glucagon at a bolus dose of 200 µg/kg as an intramuscular/subcutaneous/slow IV route. In refractory cases, continuous infusion can be given at a dose of 10–20 µg/kg/h. Reports of severe hyponatremia have been reported with glucagon. Other rare side effects reported include thrombocytopenia and necrolytic migratory erythema. In our case, glucagon bolus followed by infusion up to 20 µg/kg/h was given without side effects.
Octreotide is a long-acting somatostatin analog, administered in IV or subcutaneous intermittent doses or continuous infusion. Doses of 1 µg/kg/h up to 10 µg/kg/h have been reported in congenital hyperinsulinism and insulin overdose in adult patients.[9,10] In our case, we used octreotide to counter any endogenous insulin release due to dextrose boluses or infusions. In our patients, we started octreotide infusion at 10 µg/kg/h along with glucagon infusion.
These patients require meticulous monitoring for volume overload, and the use of diuretics may be warranted. Diazoxide therapy is commonly used for hyperinsulinemic hypoglycemia.[11] However, its use in exogenous insulin overdose is limited. We used it as a desperate measure in an attempt to attain early euglycemia in the interest of the long-term neurocognitive outcome.
Glucocorticoids have been used in treating refractory hyperinsulinemic hypoglycemia.[12] Since glucocorticoids cause insulin resistance, it can function as a true antidote. Glucocorticoids have the greatest potential benefit for patients who respond poorly to IV glucose. However, due to potential side effects, its use is restricted. The proposed mechanism is the stimulation of gluconeogenesis and reduced glucose utilization. Short-acting glucocorticoids are preferred; however, case reports of using long-acting preparations also exist.[5] These are particularly useful where fluid overload and pulmonary edema are a concern. The chance of infection is very less at these doses. In our patients, both hydrocortisone and methylprednisolone were used. Stat doses of hydrocortisone were given at a dose of 10 mg/kg/day once or twice, but in view of persistent hypoglycemia, long-acting preparations were considered. It was given earlier for twin 2 compared to twin 1. In view of hyperglycemia following the first dose at 2 mg/kg/day of methylprednisolone, doses of 1 mg/kg/day were used for subsequent doses.
Excision of subcutaneous fat at the injection site has been shown to drastically reduce dextrose infusion rates but was not done for our patients since the injection site could not be identified.
Permanent neurological damage is seen in 25–50% of neonates with severe hypoglycemia.[13] The changes include gyral atrophy, reduced white matter myelination and cortical cerebral atrophy. In our case, both the twins had severe neurodevelopmental impairment associated with radiological changes after three months and are being followed up at their native place.
CONCLUSION
We have successfully diagnosed and treated preterm twins with refractory hypoglycemia due to exogenously administered insulin. Key highlights of this case include prolonged duration of hypoglycemia despite administration of short-acting insulin, cautious use of high GIR beyond glucose oxidation rates, judicious use of second-line agents, and earlier initiation of glucocorticoids with a negative septic screen.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Recommendations from the Pediatric Endocrine Society for evaluation and management of persistent hypoglycemia in neonates, infants, and children. J Pediatr. 2015;167:238-45.
- [CrossRef] [PubMed] [Google Scholar]
- Features, prevention and management of acute overdose due to antidiabetic drugs. Drug Saf. 1993;9:218-29.
- [CrossRef] [PubMed] [Google Scholar]
- Electrolyte disorders following massive insulin overdose in a patient with type 2 diabetes. Intern Med. 2000;39:55-7.
- [CrossRef] [PubMed] [Google Scholar]
- ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Carbohydrates. Clin Nutr. 2018;37:2337-43.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term non-surgical therapy of severe persistent congenital hyperinsulinism with glucagon. Horm Res. 2008;70:59-64.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of continuous intravenous glucagon on glucose requirements in infants with congenital hyperinsulinism. JIMD Rep. 2019;45:45-50.
- [CrossRef] [PubMed] [Google Scholar]
- Experience with intravenous glucagon infusions as a treatment for resistant neonatal hypoglycemia. Arch Pediatr Adolesc Med. 2002;156:999-1004.
- [CrossRef] [PubMed] [Google Scholar]
- Continuous infusion of glucagon induces severe hyponatremia and thrombocytopenia in a premature neonate. Pediatrics. 2001;107:595-7.
- [CrossRef] [PubMed] [Google Scholar]
- Octreotide for the treatment of intentional insulin aspart overdose in a non-diabetic patient. CJEM. 2018;20:643-7.
- [CrossRef] [PubMed] [Google Scholar]
- Octreotide for the treatment of hypoglycemia after insulin glargine overdose. J Emerg Med. 2013;45:194-8.
- [CrossRef] [PubMed] [Google Scholar]
- Hyperinsulinemic hypoglycemia in infancy: Current concepts in diagnosis and management. Indian Pediatr. 2015;52:1051-9.
- [CrossRef] [PubMed] [Google Scholar]
- Role of steroids in refractory hypoglycemia due to an overdose of 10,000 units of insulin glargine: A case report and literature review. AACE Clin Case Rep. 2017;4:e70-4.
- [CrossRef] [Google Scholar]
- Research advances in neonatal hypoglycemic brain injury. Transl Pediatr. 2012;1:108-15.
- [Google Scholar]







