Translate this page into:
A study on normalization of hypothyroxinemia in neonates below 34 weeks of gestation

*Corresponding author: Seema Gaonkar, Department of Pediatrics and Neonatology, Cloudnine Hospital, Bengaluru, Karnataka, India. seema.mgaonkar@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Gaonkar S, Shenoi A, Sathyanarayana SO, Namachivayam AK, Raja DM, Rao N. A study on normalization of hypothyroxinemia in neonates below 34 weeks of gestation. J Pediatr Endocrinol Diabetes 2022;2:56-62.
Abstract
Objectives:
The aim of the study was to estimate the time required for normalization of hypothyroxinemia of prematurity in neonates below 34 weeks of gestation.
Material and Methods:
A retrospective study was conducted in neonates born below 34 weeks of gestation, between January 2015 and December 2016. Data were collected on free thyroxine (fT4) and thyroid-stimulating hormone (TSH) levels, tested on days 3, 14, 28, and 42. Gestational age, birth weight, use of antenatal steroids, mechanical ventilation, and various preterm morbidities, along with development at 18 months of corrected age, were comparatively analyzed in neonates with and without hypothyroxinemia. The median time for normalization of fT4 in all these variables was estimated.
Results:
On day 3, low fT4 was noted in 69 (37.7%) out of 183 neonates born below 34 weeks of gestation; all had normal TSH levels. Hypothyroxinemia showed statistically significant association with gestational age, birth weight, antenatal steroid use, respiratory distress syndrome, invasive ventilation, shock, sepsis, patent ductus arteriosus (PDA), anemia during stay in neonatal intensive care unit, and development at 18 months. Median time for normalization was 14 days in most of the neonates, and 28 days in those with <28 weeks of gestational age, weight of <1000 g and with shock, anemia, and PDA. Two infants with hypothyroxinemia received therapy with levothyroxine at 6 weeks for a short duration, as TSH was high.
Conclusion:
Hypothyroxinemia of prematurity takes 14–28 days to normalize based on maturity, weight, and illnesses. This study recommends serum fT4 testing at 2 weeks of life, provided congenital hypothyroidism was ruled out by 3–4 days of life, using direct blood spot card metabolic screening.
Keywords
Hypothyroxinemia of prematurity
Low birth weight
Thyroxine
Thyroid-stimulating hormone
Normalization of free thyroxine
INTRODUCTION
The thyroid function in preterm neonates differs from term neonates. The immaturity of the hypothalamic-pituitary-thyroid (HPT) axis in preterm neonates leads to blunted thyroid-stimulating hormone (TSH) surge and low free thyroxine (fT4), a condition known as “transient hypothyroxinemia of prematurity” (THOP).[1-3] THOP is mostly benign but should be differentiated from congenital hypothyroidism (CH) at the earliest. CH demands immediate treatment to prevent neurodevelopmental delay,[3,4] and levothyroxine supplementation should be initiated within 2 weeks of life.
Possibilities of both false-negative and false-positive thyroid profiles exist in preterm neonates. When and how to screen for thyroid dysfunction in preterm neonates is always debatable. At present, no universally accepted guidelines are available regarding screening and management of hypothyroxinemia in preterm infants,[5] though there are number of guidelines on CH. The European Society for Pediatric Endocrinology (ESPE) recommended that all preterm infants should undergo repeat screening, as the current screening program may not identify CH in premature infants due to delayed elevation of TSH.[6] The 2020–2021 Consensus Guidelines Update – an ENDO-European Reference Network Initiative endorsed by the ESPE and the European Society for Endocrinology – further endorses that in a group of special categories of neonates including premature, low birth weight, and sick babies who are at risk of CH not detected by initial neonatal screening, a repeat strategy including collection of a second specimen at 10–14 days of age may be considered.[7] The Indian Society for Pediatric and Adolescent Endocrinology (ISPAE) guidelines on CH in 2018 recommended screening every newborn including preterm and low birth weight, ideally at 48–72 h of age and for milder elevation of screen TSH, a second screening TSH at 7–10 days.[8] There are no further updates on Indian guidelines for newborn thyroid screening. However, a clinical practice algorithm published in 2021 on newborn screening for CH based on the ISPAE guidelines, recommended repeating the thyroid hormone profile in preterm babies after 2–4 weeks or at the time of discharge whichever was earlier.[9] An Indian study published in 2021 advocated that all preterm should be retested at term or before discharge, irrespective of the initial thyroid screening results.[10]
The current retrospective analysis was conducted to estimate the proportion of preterm neonates who persisted with a low fT4 level and to analyze the median time needed for normalization of fT4 since birth. This study tried to estimate impact of various clinical variables such as gender, gestational age, birth weight, preterm morbidities, and use of antenatal steroids on the time needed to normalize fT4. In addition, the development of infants with hypothyroxinemia was compared with preterm infants who had a normal thyroid profile.
MATERIAL AND METHODS
This single-center retrospective study was conducted at Cloudnine Hospital, OAR branch, Bengaluru, a tertiary hospital, after obtaining ethics committee approval. Neonates admitted in neonatal intensive care unit (NICU) between January 2015 and December 2016 were included in this study. Free T4 (fT4) and TSH levels of the participants were collected from the medical records. During the study period, as per NICU protocol, serum fT4, and TSH were tested in neonates <34 weeks of gestation on day 3. Dry blood spot (DBS) card test as a part of newborn metabolic screening test was done on day 3. Preterm neonates with fT4 below <15.5 pmol/L and normal TSH (<20 µlU/mL) on day 3 of life were considered to have THOP.[11] Neonates who had normal fT4 (≥15.5 pmol/L) and normal TSH on day 3 were considered as No-THOP. Neonates with major congenital anomalies (neural tube defects, cyanotic cardiac defects, abdominal wall defects, and/or chromosomal anomalies), who expired within 1 month were excluded from the study [Figure 1]. In neonates with hypothyroxinemia on day 3, serum fT4 and TSH were retested till fT4 normalized at either 14, 28, or 42 days, whichever was the earliest. If TSH was high anytime, irrespective of fT4 value, the neonate was evaluated for CH, as per standard protocol and supplemented with levothyroxine if needed.
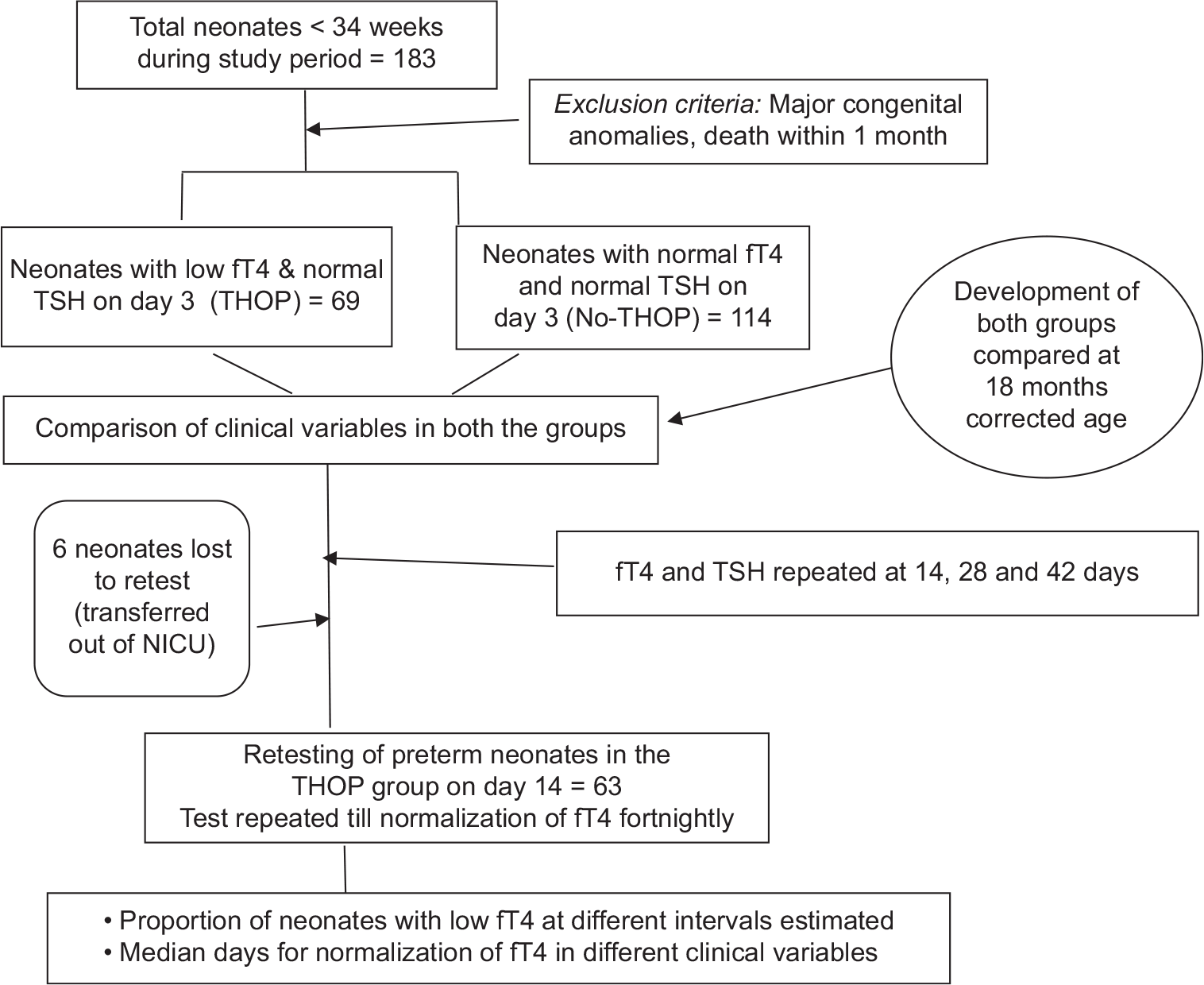
- Flowchart on study design.
Chemiluminescence Microparticle Immune Assay (Architect, Abbott Diagnostics) was used for the assessment of thyroid profile. Free T4 <15.5 pmol/L and TSH levels >20 µlU/mL on the first 14 days, and fT4 <12 pmol/L and TSH levels >10 µIU/mL after 14 days were considered as abnormal thyroid profile.[11] All neonates with abnormal thyroid hormone profiles were reviewed by the pediatric endocrinologist as per the hospital protocol.
Clinical variables were also collected from the medical records such as gender, gestational age, birth weight, use of antenatal steroids, invasive ventilation, morbidities such as respiratory distress syndrome (RDS), sepsis, anemia, shock, intraventricular hemorrhage (IVH), and hemodynamically significant-patent ductus arteriosus (HS-PDA). Preterm neonates were stratified into distinct categories of gestation and birth weight (as per the World Health Organization criteria) as follows: Gestational age categories being extreme preterm (<28 weeks), very preterm (28+0 to 31+6 weeks), moderate preterm (32+0 to 33+6 weeks), and birth weight categories being extreme low birth weight (ELBW, <1000 g), very low birth weight (VLBW, 1000–1499 g), and low birth weight (LBW, 1500–2499 g).[12] RDS was diagnosed on clinical and radiological basis, requiring either invasive or non-invasive ventilation, with or without surfactant administration. Sepsis was diagnosed based on either positive blood culture or clinical criteria. If there was requirement of packed red cell transfusion or inotropes during hospital stay, a diagnosis of anemia and shock was considered, respectively. IVH of Grade 2 and above (Volpe classification) or bilateral involvement was included as IVH. HS-PDA was defined a significant PDA on clinical and echocardiographic findings and treated with medical and/or surgical measures. Based on the hospital protocol, steroids were administered to mothers with threatened preterm birth. All these clinical variables were compared between the THOP and No-THOP groups. The proportion of neonates who had persistent low fT4 levels on days 14, 28, and 42 was estimated. Median time for normalization of fT4 in preterm neonates with THOP was estimated and an association with clinical variable was examined.
As a part of the hospital protocol, the development of all preterm neonates below 34 weeks of gestation was performed during follow-up at 18 months of corrected age. The developmental assessment was done by a team including a developmental pediatrician and physiotherapist using the “Developmental Assessment Scales for Indian Infant” (DASII) for direct assessment and “Denver-2 developmental test” for teleassessment. Developmental outcome was compared between the THOP and the No-THOP groups (67 infants were assessed; two died before the age of assessment). A DASII score ≤85 in mental or motor or both domains was considered as having developmental delay.
Statistical analysis
Collected data were entered into Excel software and analyzed using R software version 4.0.0. Categorical variables were presented as count and percentage. The association between fT4 and clinical parameters was done using Chi-square test. Duration of normalization was presented as median and range. Mann–Whitney U-test and Kruskal–Wallis test were used to compare the median between two groups and more than 2 groups, respectively. P < 0.05 was considered statistically significant.
RESULTS
One hundred and eighty-three neonates were born below 34 weeks of gestation during the study period. Low serum fT4 was noted in 69 (37.7%) of these neonates on day 3; all had normal TSH (these were considered as THOP group). The remaining 114 preterm neonates had normal fT4 and normal TSH (considered as No-THOP group). Gender distribution was equal in hypothyroxinemia neonates, with no significant difference in both THOP and No-THOP groups [Table 1].
| Clinical variables | fT4 level (pmol/L) | P-value | |
|---|---|---|---|
| No-THOP (n=114), number (%) | THOP (n=69), number (%) | ||
| Gender | |||
| Male (n=101) | 66 (57.9) | 35 (50.7) | 0.361 |
| Female (n=82) | 48 (42.1) | 34 (49.3) | |
| Gestation category | |||
| <28 weeks (n=12) | 0 (0.0) | 12 (17.4) | <0.001* |
| 28–31+6 weeks (n=34) | 11 (9.6) | 23 (33.3) | |
| 32–33+6 weeks (n=137) | 103 (90.4) | 34 (49.3) | |
| Birth weight (g) | |||
| <1000 (n=15) | 0 (0.0) | 15 (21.7) | <0.001* |
| 1000–1499 (n=35) | 14 (12.3) | 21 (30.4) | |
| 1500–2499 (n=127) | 94 (82.5) | 33 (47.8) | |
| >2500 (n=6) | 6 (5.3) | 0 (0.0) | |
| Weight for gestational age | |||
| SGA (n=12) | 4 (3.5) | 8 (11.6) | 0.077 |
| AGA (n=170) | 109 (95.6) | 61 (88.4) | |
| LGA (n=1) | 1 (0.9) | 0 (0.0) | |
| Antenatal steroid use | |||
| Not given (n=48) | 35 (30.7) | 13 (18.8) | 0.004* |
| Partially given (n=77) | 53 (46.5) | 24 (34.8) | |
| Given full course (n=58) | 26 (22.8) | 32 (46.4) | |
| RDS (n=76) | 29 (25.4) | 47 (68.1) | <0.001* |
| Invasive ventilation (n=24) | 6 (5.3) | 18 (26.1) | <0.001* |
| IVH (n=5) | 1 (0.9) | 4 (5.8) | 0.068* |
| Shock (n=9) | 0 (0.0) | 9 (13.0) | <0.001* |
| Sepsis (n=52) | 18 (15.8) | 34 (49.3) | <0.001* |
| HS-PDA (n=11) | 0 (0.0) | 11 (15.9) | <0.001* |
| Anemia of prematurity (n=25) | 2 (1.8) | 23 (33.3) | <0.001* |
| Developmental delay | |||
| No delay (n=161) | 106 (93.0) | 55 (79.7) | 0.015* |
| Delay present (n=20) | 8 (7.0) | 12 (17.4) | |
| Died before 18 months CA (n=2) | 0 (0.0) | 2 (2.9) | |
THOP: Transient hypothyroxinemia of prematurity, fT4: Free thyroxine, SGA: Small for gestational age, AGA: Appropriate for gestational age, LGA: Large for gestational age, HS-PDA: Hemodynamically significant patent ductus arteriosus, CA: Corrected age, IVH: Intraventricular hemorrhage, RDS: Respiratory distress syndrome. *Statistically significant (P<0.05)
As shown in [Table 1], all 12 neonates born below 28 weeks of gestation (extreme preterm) had low fT4 on day 3. Similarly, all 15 neonates with birth weight <1000 g had hypothyroxinemia on day 3. A lesser proportion of THOP was noted with higher gestation and/or larger birth weight categories. There were significant differences in neonates with and without hypothyroxinemia in both gestation and birth weight categories, but not with weight for gestation [Table 1]. Hypothyroxinemia on day 3 had RDS, invasive ventilation, shock, sepsis, HS-PDA, and anemia in comparison to those with No-THOP [Table 1]. The delay in development at 18 months was seen in 12 (17.9%) subjects with THOP (out of 67 assessed) and 8 (7%) subjects with No-THOP (114 were assessed) and this was statistically significant [Table 1].
Rescreening
Sixty-three neonates were retested for fT4 and TSH on day 14; six were not available. Thirteen (20%) had persistent low fT4 on day 14; it was observed that all of them were below 32 weeks of gestation and below 1500 g birth weight. These neonates got rescreened fortnightly till normalization of fT4 was observed. At day 28 and day 42, 5 (8%) and 2 (3%) preterm neonates persisted to have low fT4 [Figure 2]. The two neonates had persistently low fT4 at day 42, showed high serum TSH (10–12 µlU/mL), and hence received short duration of levothyroxine (for around 8 weeks). They were followed up by the pediatric endocrinologist until 2 years of age; both had normal thyroid function. Rest of the neonates had normal TSH levels in all the retests.

- Proportion of neonates with low free T4 at different time intervals.
The median duration for normalization of fT4 was 28 days in extreme preterm and extreme low birth weight neonates and in sick neonates who had shock, anemia, and HS-PDA [Table 2]. It was 14 days in higher gestational age (28– 31+6 weeks and 32–33+6 weeks) and larger birth weight categories (1000–1499 g, 1500–2499 g, and >2500 g) and in neonates with RDS, invasive ventilation, and sepsis; the association was statistically significant.
| Clinical variables | THOP neonates (n=63) | Median (range) | P-value |
|---|---|---|---|
| Gestational age (weeks) | <28 (n=12) | 28.0 (14–42) | <0.001* |
| 28–31 (n=20) | 14.0 (14–28) | ||
| 32–34 (n=31) | 14.0 (14–14) | ||
| Birth weight (g) | <1000 (n=15) | 28.0 (14–42) | <0.001* |
| 1000–1499 (n=19) | 14.0 (14–42) | ||
| 1500–2499 (n=29) | 14.0 (14–14) | ||
| Weight for gestation | SGA (n=7) | 14.0 (14–42) | 0.556 |
| AGA (n=56) | 14.0 (14–42) | ||
| LGA (0) | - | ||
| Antenatal steroid use | Not given (n=10) | 14.0 (14–42) | 0.732 |
| Partially given (n=21) | 14.0 (14–42) | ||
| Given full course | 14.0 (14–42) | ||
| (n=32) | |||
| RDS | Present (n=43) | 14.0 (14–42) | 0.007* |
| Absent (n=20) | 14.0 (14–14) | ||
| Invasive ventilation | Present (n=17) | 14.0 (14–42) | 0.001* |
| Absent (n=46) | 14.0 (14–28) | ||
| Sepsis | Present (n=33) | 14.0 (14–42) | 0.011* |
| Absent (n=30) | 14.0 (14–42) | ||
| HS-PDA | Present (n=11) | 28.0 (14–42) | <0.001* |
| Absent (n=52) | 14.0 (14–42) | ||
| Anemia of prematurity Shock | Present (n=23) | 28.0 (14–42) | <0.001* |
| Absent (n=40) | 14.0 (14–28) | ||
| Present (n=9) | 28.0 (14–42) | <0.001* | |
| Absent (n=54) | 14.0 (14–42) | ||
| IVH | Present (n=4) | 21.0 (14–42) | 0.124 |
| Absent (n=59) | 14.0 (14–42) |
THOP: Transient hypothyroxinemia of prematurity, fT4: free thyroxine, RDS: Respiratory distress syndrome, HS-PDA: Hemodynamically significant patent ductus arteriosus, IVH: Intraventricular hemorrhage, AGA: Appropriate for gestation, SGA: Small for gestation, LGA: Large for gestation. *Statistically significant (P<0.05)
DISCUSSION
THOP is an entity to be considered, when a preterm neonate has low fT4 level, with a normal TSH level. It is always crucial to ensure that TSH is within the normal range as early as possible (ideally within 3–4 days of life) and rule out CH before considering THOP as the diagnosis. Hypothyroxinemia of prematurity results from the immaturity of the HPT axis in preterm neonates; immature synthesis and metabolism of thyroid hormones; and preterm morbidities also add to it.[2] The most vulnerable population for hypothyroxinemia are the preterm neonates born at extreme low gestation (<28 weeks), extreme low birth weight (<1000 g), and the very sick preterm neonates with a difficult NICU stay. Most of them grow in weight, maturity, and health over a period of time and their thyroid function normalizes. However, there is a possibility of few of them, remaining with continued low fT4 or gradually rising TSH, suggestive of hypothyroidism, transient, or permanent. Hence, rescreening the thyroid profile of these neonates is an essential part of NICU care. The time needed for normalization of fT4 level varies with gestational age, birth weight, and preterm morbidities – RDS, sepsis, anemia, shock, IVH, PDA, etc.
There are only a limited number of studies on hypothyroxinemia in preterm neonates in Indian scenario. Although there are a number of guidelines on CH, there are no specific guidelines available for hypothyroxinemia of prematurity – when to screen and rescreen, when and whether to treat THOP. The present study was intended to estimate the prevalence of hypothyroxinemia in preterm babies, to analyze the proportion which persisted with low fT4 at different intervals and the median duration needed for its normalization and whether THOP is influenced by clinical variables.
In the present study, hypothyroxinemia was present in one-third (37.7%) of preterm neonates tested. THOP was more pronounced with the extreme preterm and extreme low birth weight population. Preterm infants who suffered various morbidities such as RDS, sepsis, shock, anemia, and HS-PDA had significant THOP compared to healthier neonates. The use of antenatal steroids and invasive ventilation also had significant association with THOP. These observations indicate that vulnerable sick preterm neonates are at a higher risk of THOP. Sickness adds to suppression of thyroid axis. There are studies to support these observations, which highlight the association of prolonged THOP in neonates with RDS, sepsis, anemia, shock, and HS-PDA.[13] In one of the studies, it was concluded that THOP in the first week of life may be a poor prognostic factor for neonatal morbidities such as RDS, mechanical ventilation, and length of hospitalization.[14]
On fortnightly rescreening, a reduction in the proportion of neonates with hypothyroxinemia was noted. Neonates who remained with low fT4 on day 14 were all below <32 weeks of gestation and birth weight <1500 g [Figure 2]. At 42 days, two of them still had low fT4 with high TSH of 10–12 µlU/mL and were treated with 8 weeks levothyroxine. Both of them were extremely preterm newborns with a birth weight of <1000 g and sick with multiple morbidities in the NICU. Over a period of time, their thyroid function normalized, clinical condition improved, and they were followed up by the pediatric endocrinologist for 2 years. Extreme low gestation and extreme low birth weight are independent risk factors for prolonged hypothyroxinemia and adverse developmental outcome. This group is susceptible to several comorbidities in the NICU that can impact outcomes further. Thyroid hormone replacement in hypothyroxinemia of prematurity is always questionable, as it is not clear whether it improves morbidities and neurodevelopmental outcome.[3] Some studies have recommended against the supplementation of levothyroxine for neonates with low thyroid hormone levels unless they have elevated TSH levels.[15,16] A study done in Korea, suggested levothyroxine supplementation if there is persistent low fT4 during the first 3 weeks.[2]
When the median duration of normalization was associated with clinical variables, neonates with <28 weeks of gestation, birthweight <1000 g and sick neonates with shock, anemia, and PDA required longer time to normalize than neonates with other morbidities and higher gestational age and birth weight. In an observational study, fT4 level normalized by 7 weeks of birth in preterm neonates.[17] An Indian study also had observed that hypothyroxinemia of prematurity is transient and spontaneous recovery occurs by 6–8 weeks of age, which supports observations made in the present study.[18] The current study also observed that preterm neonates mature and stabilize to have normal thyroid function by 2–4 weeks of age. Hence, this study recommends that the estimation of serum thyroid function tests can be postponed to 2 weeks of life, instead on day 3 after birth. However, it is essential to rule out CH at the earliest, within 3–4 days of life, which was practiced with a DBS card test, as an integral part of the newborn screening program. There is a possibility of delayed surge of TSH in preterm neonates due to immature HPT axis and also due to sickness, leaving them at risk of missing the diagnosis of hypothyroidism. Testing thyroid profile at 2 weeks of age in preterm neonates would help in detection of such hypothyroidism, which would have been missed in the first few days. Postponing the serum blood test to 2 weeks can reduce the blood loss, sampling stress, cost, and parental anxiety in early neonatal period for THOP.
Few studies recommend routine repetition of thyroid function in all preterm neonates even if initial thyroid profile is normal.[19] The Section on Endocrinology and Committee on Genetics recommends that routinely NBS for hypothyroidism at 2 time periods can detect CH in approximately 10% of the affected infants only by collecting a second specimen. Most of these newborns were LBW or VLBW, with mild or delayed TSH elevations.[20] In the present study, only neonates with abnormal thyroid profile were rescreened, but not those with normal initial fT4 and TSH. This was done as per the hospital protocol during the study period which is one of the limitations of the study.
Development at 18 months of corrected age was assessed for all the infants born <34 weeks of gestation during the study period, as per the developmental follow-up program of the center. Statistical correlation was noted between THOP and developmental delay at 18 months of age in the present study. There is always a debate on whether THOP affects the development in preterm infants. THOP is more pronounced in vulnerable groups with extreme gestation, extreme low birth weight, and severe morbidities, each one of them could be an individual risk factor for the adverse development. Hence, we could not establish the causal relationship between THOP and delay in development. We need to have large-scale studies, to ascertain a causal effect between THOP and delayed development. Only a few studies have reported similar mean mental and psychomotor developmental index scores in preterm infants with and without THOP at 18–24 months of corrected age.[21-23] Two infants who received levothyroxine for a short duration in this study did not have developmental delay, though both were most vulnerable and sick preterm neonates. However, the number is too small to come to any conclusion. Opinions differ as one of the studies in premature infants with hypothyroxinemia suggested that infants >27 weeks of gestation do not appear to benefit from levothyroxine therapy, but short-term levothyroxine supplementation in infants born before 27 weeks of gestation may be important to diminish morbidity and to improve neurodevelopmental outcome.[24] Many studies concluded no association between THOP and adverse neurodevelopmental outcomes.[25]
There are many points which increase the strength of this study: There is less data collection bias as data were collected over a period of 2 years using a standard uniform protocol in a single center. Developmental assessment at 18 months was a part of the study. Moreover, the program had a follow-up of 99% for assessment (direct and teleassessment).
The study had its limitations too. The reference values used for thyroid profile are only from one published data. The reference values for thyroid hormone profile were not individualized for each week of gestation, as that were not practiced at the hospital during the study period to define THOP. In the present analysis, only neonates with low fT4 had the retesting, not all preterm neonates were retested. We recommend rescreening all preterm neonates irrespective of their initial thyroid profile, to identify neonates with delayed TSH rise. Developmental outcome was mentioned only as delayed or normal and was not interpreted for each domain and the degree of delay. This was done for easy interpretation, as development was only a part of the study.
CONCLUSION
Nearly 14–28 days are needed for normalization of hypothyroxinemia of prematurity, which is prolonged in extreme preterm, extreme low birth weight, and sick neonates. This study suggests that initial serum fT4 testing for preterm babies should be done at 2 weeks of life, provided high TSH is ruled out with DBS card performed at 3–4 days of life. The national neonatology and endocrinology bodies should work together to prepare nomograms for premature babies of different gestational ages and formulate guidelines for screening and management of THOP.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- High incidence of thyroid dysfunction in preterm infants. J Korean Med Sci. 2009;24:627-31.
- [CrossRef] [PubMed] [Google Scholar]
- Thyroid dysfunction in very low birth weight preterm infants. Korean J Pediatr. 2015;58:224-9.
- [CrossRef] [PubMed] [Google Scholar]
- Neurobehavioral deficits in premature graduates of intensive care potential medical and neonatal environmental risk factors. Pediatrics. 2001;108:1339-48.
- [CrossRef] [PubMed] [Google Scholar]
- Thyroid dysfunction in preterm infants born before 32 gestational weeks. BMC Pediatr. 2019;19:391.
- [CrossRef] [PubMed] [Google Scholar]
- European society for paediatric endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. J Clin Endocrinol Metab. 2014;99:363-84.
- [CrossRef] [PubMed] [Google Scholar]
- Congenital hypothyroidism: A 2020-2021 consensus guidelines update an ENDO-European reference network initiative endorsed by the European society for pediatric endocrinology and the European society for endocrinology. Thyroid. 2021;31:387-419.
- [CrossRef] [PubMed] [Google Scholar]
- Newborn screening guidelines for congenital hypothyroidism in India: Recommendations of the Indian society for pediatric and adolescent endocrinology (ISPAE) Part I: Screening and confirmation of diagnosis. Indian J Pediatr. 2018;85:440-7.
- [CrossRef] [PubMed] [Google Scholar]
- Newborn Screening for Congenital Hypothyroidism (NBS-CH) Clinical Practice Guideline Algorithm Based on ISPAE. CH-2018 Guidelines.
- [Google Scholar]
- Congenital hypothyroidism in Indian preterm babies-screening, prevalence, and aetiology. Pediatr Endocrinol Diabetes Metab. 2021;27:82-6.
- [CrossRef] [PubMed] [Google Scholar]
- Developmental trends in cord and postpartum serum thyroid hormones in preterm infants. J Clin Endocrinol Metab. 2004;89:5314-20.
- [CrossRef] [PubMed] [Google Scholar]
- PO7-Disorders of Short Gestation and Low Birth Weight; Chapter 14th International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10), Version for.
- [Google Scholar]
- The factors associated with transient hypothyroxinemia of prematurity. BMC Pediatr. 2021;21:344.
- [CrossRef] [PubMed] [Google Scholar]
- Serum thyroid hormone levels in preterm infants born before 33 weeks of gestation and association of transient hypothyroxinemia with postnatal characteristics. J Pediatr Endocrinol Metab. 2010;23:899-912.
- [CrossRef] [PubMed] [Google Scholar]
- Hypothyroxinemia in the preterm infant: The benefits and risks of thyroxine treatment. J Pediatr. 2001;139:182-8.
- [CrossRef] [PubMed] [Google Scholar]
- Neonatal thyroid disorders. Arch Dis Child Fetal Neonatal Ed. 2002;87:F165-71.
- [CrossRef] [PubMed] [Google Scholar]
- Does hypothyroxinemia of preterm neonates persist beyond 7 weeks of life? Indian J Pediatr. 2019;86:686-91.
- [CrossRef] [PubMed] [Google Scholar]
- Hypothyroxinemia in preterm neonates: Not always hypothyroidism. Indian J Pediatr. 2019;86:671-2.
- [CrossRef] [PubMed] [Google Scholar]
- Thyroid function in the very low birth weight newborn: Rescreen or reevaluate? J Pediatr. 2002;140:287-9.
- [CrossRef] [PubMed] [Google Scholar]
- Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics. 2006;117:2290-303.
- [CrossRef] [PubMed] [Google Scholar]
- Transient hypothyroxinemia of prematurity does it have clinical relevance? Indian Pediatr. 2012;49:703-4.
- [CrossRef] [PubMed] [Google Scholar]
- Neurodevelopmental evaluation of very low birth weight infants with transient hypothyroxinemia at corrected age of 18-24 months. Indian Pediatr. 2012;49:711-5.
- [CrossRef] [PubMed] [Google Scholar]
- Postnatal thyroid hormones for preterm infants with transient hypothyroxinemia. Cochrane Database Syst Rev. 2007;2007:CD005945.
- [CrossRef] [Google Scholar]
- Hypothyroxinemia of prematurity: Rite of passage or therapeutic necessity? Tex Med. 2000;96:60-3.
- [Google Scholar]
- Lack of association between hypothyroxinemia of prematurity and transient thyroid abnormalities with adverse long term neurodevelopmental outcome in very low birth weight infants. PLoS One. 2019;14:e02222018.
- [CrossRef] [PubMed] [Google Scholar]







