Translate this page into:
Type 1 diabetes self-care in urban schools in India

*Corresponding author: Sirisha Kusuma Boddu, Department of Pediatric Endocrinology, Rainbow Children’s Hospital, Hyderabad, Telangana, India. sirisuma@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Virmani A, Boddu SK, Sarda A, Shukla R, Puri S, Chhabra M, et al.: Type 1 diabetes self-care in urban schools in India. J Pediatr Endocrinol Diabetes 2021;1:8-13.
Abstract
Objectives:
Children with type 1 diabetes (T1D) need a supportive, non-stigmatizing school environment for self-care activities such as checking blood glucose (BG) and taking pre-meal insulin. Data about T1D self-care in schools in developing countries are scarce. We looked at diabetes self-care activities at school, and attitudes of school staff toward diabetes care.
Material and Methods:
We interviewed, over an 8-week period, consecutive patient-parent dyads attending T1D clinics in North (Delhi, Gurgaon, and Kanpur), West (Aurangabad), and South (Hyderabad) India.
Results:
We received responses from 397 patients, 51% of boys. Mean age was 11.7 years (SD: 3.7), mean age at diagnosis 7.2 years (SD: 3.7), and mean diabetes duration 4.5 years (SD: 3.5). A majority (69.8%) were attending private (fee paying) schools (PS) and the rest were studying at government (subsidized/free) schools (GS). More than half of the parents had high educational status: graduate or more (mothers: 52.1%, fathers: 56.9%). Parents visited school daily in 17.1%, significantly more if they had high educational status and if the child was <6 years. Less than half (47.4%) were administering a pre-meal insulin bolus at school (self-injection: 33%, by parent: 12.9%, and by staff: 1.5%); only 24.4% were checking BG regularly (< once per week) at school. The odds of performing diabetes self-care activities at school were significantly higher in children attending PS compared to GS (OR: 3.17, 95% CI: 1.99–5.03 for taking insulin, OR: 3.24, 95% CI: 1.75–5.98 for regular BG checking). The odds of taking insulin at school were also higher with higher parental education (OR: 2.81, 95% CI: 1.87–4.24 for mother’s education, OR: 3.02, 95% CI: 1.99–4.57 for father’s). Testing and injecting we done in classroom (26.2%); medical room (16.1%), staffroom (7.8%), or toilet (2.5%). School insisted on secrecy in 12.6%, excluded children with T1D from sports/excursions in 17.9%, refused permission for injecting in 4.3%, for testing 15.9%, and for pre-activity snack 7.6%. This non-supportive behavior was equal in PS and GS. PS had slightly better care infrastructure such as availability of glucometer (29.6% vs. 3.3%), sick room (21.7% vs. 0.3%), and dedicated nurse (9.7% vs. none).
Conclusion:
Half of our children were able to manage T1D self-care in school, as schools were often supportive, whether private or government. Parental educational status was positively associated with better care. Although self-care was better in PS and they had better infrastructure, there is much scope for improvement.
Keywords
Type 1 diabetes
Child
Diabetes Self-care
India
School support
INTRODUCTION
Children spend much of their waking hours at school, doing activities including travel to and from, engaging in studies, social interaction, eating meals and snacks, participating in sports, etc., all of which affect and can be affected by fluctuating blood glucose (BG) levels. In children and young people with type 1 diabetes (T1D), for good glycemic control and quality of life, several diabetes self-care activities are needed even at school. These can range in complexity from simply doing a finger-prick BG check and taking pre-meal insulin injection to checking BG from the continuous glucose monitoring system, calculating bolus dose, and administering it through the insulin pump; in addition to preventing and handling emergencies such as hypoglycemia and ketosis. For this, a supportive, safe, and non-stigmatizing school environment is needed.[1] Increasing prevalence of T1D appears to have reduced the stigma and fear associated with diabetes to some extent. An indicator of this positive change is the 2017 decision by the Indian Central Board of Secondary Education allowing children with TID appearing for examinations to carry eatables into the examination hall.[2] Nevertheless, quality of care and institutional support remain uneven in developing countries. While studies conducted on parental perceptions indicate suboptimal care at schools even in developed nations, data from India are extremely limited.[3]
Objectives
We aimed to look at the ground reality of the school environment, faced by children attending T1D clinics in different cities of India. We wanted to study the current T1D self-care activities in these urban schools and the parental perceptions of school support.
MATERIAL AND METHODS
We did a cross-sectional observational survey, over a period of 8 weeks, of all consecutive school-going children and adolescents with T1D and their parents attending T1D clinics in eight centers in North (Delhi, Gurgaon, and Kanpur), West (Aurangabad), and South (Hyderabad) India. During clinic visits, the authors administered a pre-approved questionnaire, in regional language as well as English. Data were collected about age, gender, duration of diabetes, treatment regimen, type of school (private/paying vs. government/free), parental educational status, diabetes self-care activities at school, and attitudes of school staff (class teacher/school nurse) toward child’s diabetes. Parental educational status was divided into low (illiterate or studied up to primary/middle school), medium (completed class 12), and high (completed graduation or higher education). Type of school and parental education were taken as surrogate markers of socioeconomic status of the family. Ethical clearance was obtained from the Institutional Ethics Committee of Rainbow Children’s Hospital, for analysis and use of anonymized data; consent was verbal and implied when agreeing to be interviewed. Assuming the prevalence of 50%, precision error of 5.5%, confidence interval 95%, design effect of 1.25, and allowing for 10% non-response rate, the computed sample size was 440. Statistical analysis was performed using SPSS version 24.0 (IBM Corp, 2016). Chi-square test was used to test association between categorical variables.
RESULTS
Of the 422 patient/parent dyads approached, 397 agreed to be interviewed. Mean age of the participants was 11.7 years (SD: ±3.7), mean age at diagnosis was 7.2 years (SD: ±3.7), and mean diabetes duration was 4.5 years (SD: ±3.5). Two-thirds (69.8%) were studying in private schools (PS) and rest in government schools (GS). Half of the parents had high education status [Table 1].
| Frequency n=397 |
Percentage | |
|---|---|---|
| Gender of the child | ||
| Boy | 204 | 51.4 |
| Age in years, mean (SD) | 11.7 (3.7) (range: 3–19.9) | |
| Age groups | ||
| <6 years | 28 | 7.0 |
| 6–11 years | 142 | 35.8 |
| >11 years | 227 | 57.2 |
| Duration of T1D in years, mean (SD) | 4.5 (3.5) (range 3 months–15.7 years) | |
| Age in years at onset of T1D, mean (SD) | 7.2 (3.7) (range 7 months–17.6 years) | |
| Mother’s education | ||
| Low (Illiterate/middle school) | 90 | 22.7 |
| Medium (High school) | 100 | 25.2 |
| High (Graduate/beyond) | 207 | 52.1 |
| Number of injection per day | ||
| 1 | 1 | 0.3 |
| 2 | 18 | 4.5 |
| 3 | 88 | 22.2 |
| 4 or more | 259 | 65.2 |
| CSII* | 31 | 7.8 |
| Type of school | ||
| Government. | 120 | 30.2 |
| Private | 277 | 69.8 |
| Taking insulin at school? | ||
| Yes | 188 | 47.4 |
| No | 209 | 52.6 |
| Checking BG regularly at school | ||
| Regularly (> once per week) | 97 | 24.4 |
| Sometimes (once per week or less) | 143 | 36.1 |
| Never | 157 | 39.5 |
| Carrying T1D ID-card to school | ||
| Always (Daily) | 155 | 39 |
| Sometimes | 58 | 14.6 |
| Does not have one | 184 | 46.3 |
T1D: Type 1 diabetes, *CSII: Continuous subcutaneous insulin infusion, BG: Blood glucose
Diabetes self-care at school
Although 87.4% of children were on multiple daily injections (MDI) and 7.8% were on continuous subcutaneous insulin infusion (CSII), less than half (n = 188, 47.4%, 95% CI: 42.1–51.9%) were taking a pre-meal insulin bolus at school. Of these, 69.7% were self-injecting, while a parent was going daily to school to give pre-lunch insulin in 27% (n = 51/188, mother in 48, father in 3); the rest 3.4% (n = 6) were helped by school nurse or class teacher. The use of NPH as basal insulin was significantly higher in those not taking insulin at school (45.9% vs. 26%, P < 0.001).
For administration of pre-lunch insulin at school, there was no statistically significant difference between the age groups: Under 6 years, 6–11 years, and >11 years. In those who received insulin injection at school, a parent administered it in all under 6 years old, while almost all children above 11 years (100/104) were self-injecting. In the age group of 6–11 years, 42.4% were self-injecting (n = 31/73), while the rest took help of a parent (n=37/73), or a school teacher or nurse (n = 5/73). BG was checked in school regularly (> once per week) only by 24.4% (95% CI: 19.8–28.2%). The odds of performing diabetes self-care activities were significantly higher in private schools when compared to GS (odds ratio: 3.17, 95% CI: 1.99–5.03 for taking insulin, odds ratio: 3.24, 95% CI: 1.75–5.98 for regular BG checks > once per week). The odds of taking insulin at school were also higher with higher parental education (mother graduate or more: OR: 2.81, 95% CI: 1.87–4.24; father graduate or more: OR: 3.02, 95% CI: 1.99–4.57), but were not affected by age or gender [Table 2 and Figure 1].
| Questions related to diabetes care (checking BG, taking insulin) at school | Percentage of “YES” answers | |||
|---|---|---|---|---|
| Private school n=277 percentage (n) |
Govt. school n=120 percentage (n) |
P value | ||
| Does child take insulin at school? | 55.6 (154) | 28.3 (34) | < 0.001 | |
| Does the child administer insulin injection him/herself at school? | 68.1 (105/154) | 76.4 (26/34) | 0.341 | |
| Does school nurse/class teacher help with diabetes care delivery? | 9.7 (27) | 0 (0) | <0.001 | |
| Is there a glucometer at your school? | 29.9 (83) | 3.3 (4) | < 0.001 | |
| Is the child’s BG checked regularly at school? | 29.9 (83) | 11.6 (14) | < 0.001 | |
| Do you think school nurse/class teacher knows how to identify and treat hypoglycemia? | 82.6 (229) | 82.5 (99) | 0.980 | |
| Does your school request you to maintain secrecy while injecting insulin or checking BG? | 13.7 (38) | 10 (12) | 0.305 | |
| Does your school prohibit the child’s involvement in activities like sports? | 18.7 (52) | 15.8 (19) | 0.486 | |
| Do you think the school is supportive overall? | 40.4 (112) | 52.5 (63) | 0.026 | |
|
Mother’s educational status high n=207 |
Mother’s educational status low/medium n=190 |
P value | ||
| Taking insulin at school | 59.4 (123) | 34.2 (65) | <0.001 | |
| Daily parental visit to school to deliver/supervise care | 24.1 (50) | 9.4 (18) | < 0.001 | |
|
Father’s educational status high n=226 |
Father’s educational status low/medium n=171 |
P value | ||
| Taking insulin at school | 58.8 (133) | 32.2 (55) | <0.001 | |
|
Boys n=204 |
Girls n=193 |
P value | ||
| Taking insulin at school | 47.5 (97) | 47.1 (91) | 0.936 | |
|
Age<6 years n=28 |
Age 6–11 years n=142 |
Age>11 years n=227 |
P value | |
| Taking insulin at school | 39.3 (n=11) | 51.4 (n=73) | 45.8 (n=104) | 0.390 |
| Daily parental visit to school | 50 (n=14) | 28 (n=40) | 6.2 (n=14) | 0.008 |
BG: Blood glucose
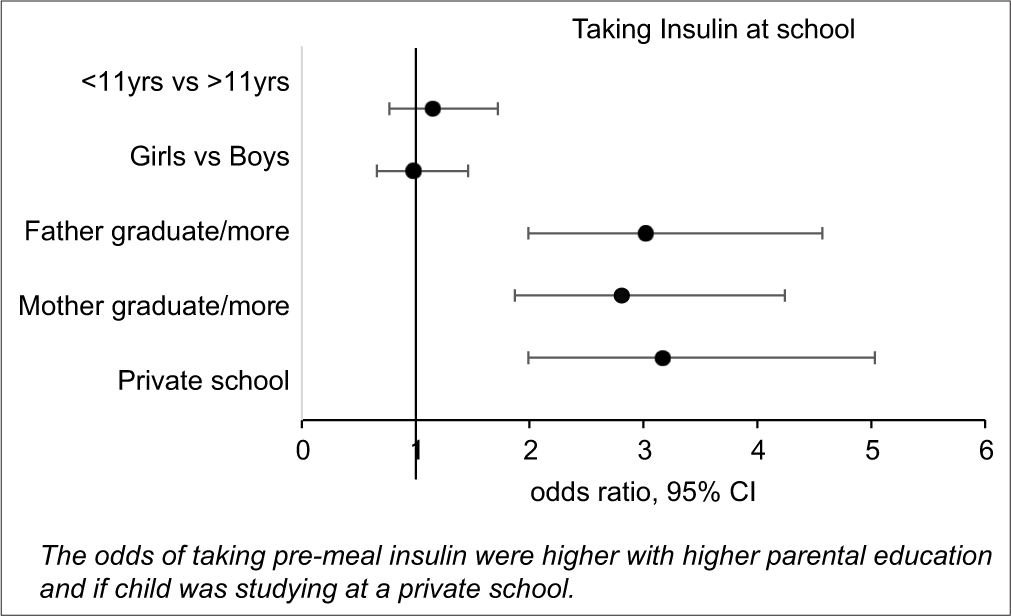
- Factors effecting pre-meal insulin administration at school.
Parents of 17.1% (95% CI: 13.3–20.6%) of children visited school daily, 74.3% few times a month to few times a year, while 8.6% had never gone to school for any diabetes-related purpose. Daily visits by one of the parents were significantly more frequent if the child was younger than 6 years (50% in <6 years vs. 28% in 6–11 years and 6.2% in >11 years, P = 0.008). Only 39% of children always carried some form of T1D identification card to school.
School infrastructure
Diabetes self-care (BG checking and insulin administration) at school was performed mostly in children’s own classroom or any empty room (49.8%), in a medical room (30.6%), in teachers’ staff room (14.8%), or in a toilet (4.8%). A fifth of parents (n = 87, 21.9%. 95% CI: 17–25%) said that their school had a glucometer, and most of these were PS. Compared to GS, PS more frequently had a separate medical room facility (PS: 60/277 vs. GS: 4/120, P < 0.001) and the availability of a nurse (PS: 27/277 vs. GS: 0/120, P < 0.001). Over half (58.9%) of the parents believed that most of the school staff knew how to identify and handle hypoglycemic episodes. This perceived awareness was similar in PS versus GS (P = 0.891) [Table 2 and Figure 2].
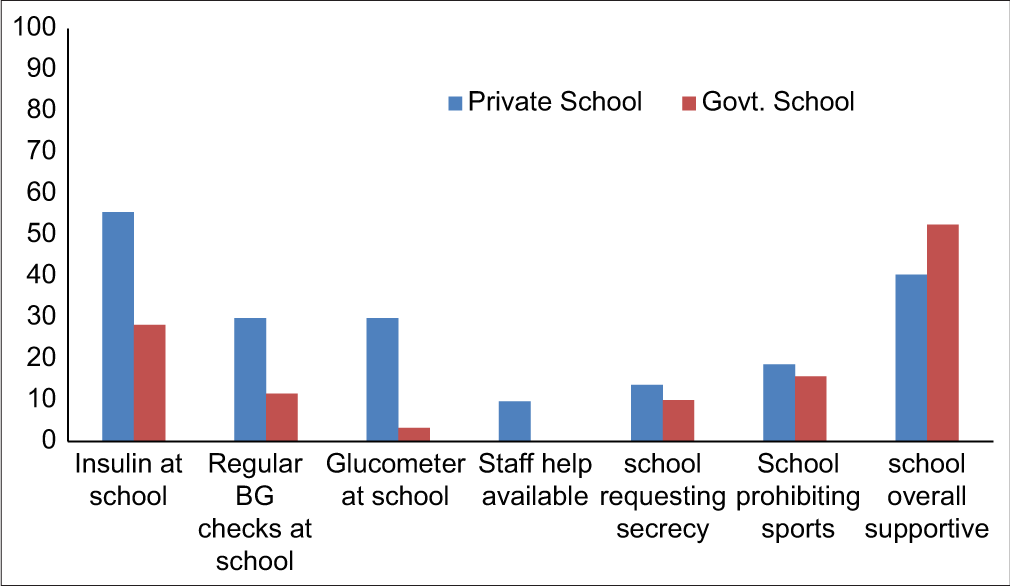
- Diabetes self-care and school attitudes: A comparison between paying (private) and free (government) schools (y-axis denotes percentage).
Support and stigma
Overall, 44% (95% CI: 39.1–48.9%) of parents felt that the school was generally supportive, 6.5% said that their child faced bullying and the school could have been more supportive in taking care of this issue, and the rest did not comment. The school wanted the child to maintain secrecy while testing/injecting in 12.6%. Overtly unsupportive attitudes such as refusing permission for testing (15.9%), injecting (4.3%), or snacking before activity (7.6%), or prohibiting participation in sports or excursions (17.9%) were less common and did not differ significantly between PS and GS. Only 3% of children had to change school because of denial of admission/open hostility, and 11 of these 12 children were studying at PS [Table 2 and Figure 2].
Teachers and child’s friends were aware of the child’s diabetes in 80.6%, only teachers knew in 15.1%, only schoolmates in 2.3%, and diabetes was kept completely secret in 2% (n = 8). Three-fourth of parents did not know if there was another child with T1D in the same school, 14.4% knew of another child with T1D but did not meet them, while 11.8% knew and met the other T1D families.
DISCUSSION
Our study group was fortuitously balanced in terms of gender and age distribution, and had good geographic spread, lending for meaningful analyses. However, it was not representative of the usual situation of T1D care in India. The children surveyed were being treated in centers with expertise in childhood diabetes, were mostly regular with follow-up, using current insulin regimens (MDI/CSII), and were willing to be interviewed. Most of the parents had informed their school about their child’s diabetes. Even the suboptimally equipped GS mostly did allow self-care to the extent possible. Nevertheless, only half of the children were testing or injecting at school, and they had to do this either themselves or with parental help.
The International Society for Pediatric and Adolescent Diabetes recommends that glycemic targets during school hours should not differ from any other setting, and optimal management of diabetes at school is a prerequisite for optimal school performance, and for the avoidance of diabetes-related short-term and log-term complications. This requires self-care activities such as BG checking, insulin administration, and identifying and treating high and low BGs.[1]
Irrespective of the country context, many children and adolescents struggle with structural, organizational, educational, and attitudinal barriers to optimal self-management of T1D in educational settings.[4-6] Developing countries like India face several additional hurdles, including poor awareness of the treating teams about T1D care, the reluctance of the team and parents to do multiple BG tests and insulin injections, societal stigma leading to parents’ unwillingness to reveal T1D to school authorities, inability of parents to visit school daily, lack of privacy, financial constraints, fear of hypoglycemia, and/or restrictions or refusal of help from school. It is unlikely that problems with treating teams’ expertise or parental insistence on maintaining secrecy regarding T1D diagnosis have contributed to inadequate diabetes self-care at school in our group.
In general, better self-care was associated with studying in PS and with higher parental educational status. Although many older children were managing to self-test and inject, many mothers, especially those with younger children (<6 years), are going daily to school for checking and injecting. Even the somewhat better infrastructure of some of the PS left much to be desired. In the absence of a well-trained school nurse (a recent concept in developing countries), the class teacher is often requested to assist or supervise the child in diabetes self-care, which could be perceived as an added burden to her/his existing responsibilities. A study from Germany surveying 678 kindergarten teachers, of whom 251 were working with children with T1D, reported deficits in three areas: Knowledge about diabetes and diabetes management, institutional support, and communication with parents and health-care professionals.[7] In addition to the knowledge gap, concerns about liability might also prevent teachers from participating in BG monitoring, or insulin injection, and might explain the active hostility of a few schools in our study group, most of them PS.[8] This was also reflected by the fact that 97% of our children who were administering insulin at school are doing so either by themselves or with the help of a parent, without any help from school staff.
Although most of our parents opined that school staff could identify and manage a hypoglycemic emergency, the available western data are to the contrary. In a large (n = 700), questionnaire-based study assessing teachers’ attitudes and perceptions, although 43% reported having a T1D student, half of them believed that overall, their school was not prepared to deal with diabetes-related emergencies.[9] Unfortunately, the scope of our study did not extend to assessing teachers and school staff, so we cannot comment on whether our parents’ confidence was justified.
Another limitation of our study is that we did not record glycemic control and thus cannot correlate our findings with diabetes control. To judge socioeconomic status, we did not directly ask for parents’ income, as replies to such questions may be unreliable. Instead, we used reliable surrogate markers: Parental education and studying in a fee-paying school. A major limitation of our work is that we could only interview parents coming to our specialized clinics, who are receiving reasonable care. We could not reach children receiving suboptimal care. Thus, our findings would not be generalizable to the situations like use of unphysiological regimens such as two doses of pre-mixed insulin, infrequent/non-existent home BG monitoring, erratic HbA1c testing, irregular follow-up, rural areas with poor baseline health infrastructure, and/or poor compliance. Unfortunately, those children would almost certainly be in a far worse situation than what we have reported.
Ours is, to the best of our knowledge, the first ever study in India intending to understand some aspects of T1D self-care in urban schools, including institutional attitudes and availability of support, and perceived challenges. More detailed studies are needed to identify gaps, especially from the schools’ perspective, strategies to close these gaps, and to explore the impact of quality of self-care at school on glycemic control and complications.
CONCLUSION
It is encouraging that about half of the children receiving care in specialized T1D clinics could manage diabetes self-care at school to some extent, especially those with better educated parents, mothers who were willing to come to school, and with schools often willing to allow such care. Diabetes self-care was better in private schools and they have slightly better infrastructure. However, overall care was suboptimal. Recognition of the problems and the contributory barriers, identifying and particularly focusing on parents with lesser education and schools with lesser infrastructure, would help diabetes care teams and policy-makers to proactively work with school managements and parents to get better care for children and adolescents with T1D.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Management and support of children and adolescents with Type 1 diabetes in school. ISPAD guidelines 2018. Pediatr Diabetes. 2018;19(Suppl 27):287-301.
- [CrossRef] [PubMed] [Google Scholar]
- Available from: http://www.cbse.nic.in/newsite/attach/circular%20on%20diabetic_2017.pdf [Last accessed on 2020 Sep 12]
- Challenges in Type 1 diabetes management in South East Asia: Descriptive situational assessment. Indian J Endocrinol Metab. 2014;18:600-7.
- [Google Scholar]
- Children with diabetes: Perceptions of supports for self-management at school. J Sch Health. 2003;73:216-21.
- [CrossRef] [PubMed] [Google Scholar]
- Barriers and facilitators to taking on diabetes self-management tasks in pre-adolescent children with Type 1 diabetes: A qualitative study. BMC Endocr Disord. 2018;18:71.
- [CrossRef] [PubMed] [Google Scholar]
- An ongoing struggle: A mixed-method systematic review of interventions, barriers and facilitators to achieving optimal self-care by children and young people with Type 1 diabetes in educational settings. BMC Pediatr. 2014;14:228.
- [CrossRef] [PubMed] [Google Scholar]
- Teachers' perspectives on children with Type 1 diabetes in German kindergartens and schools. Diabetes Spectr. 2020;33:201-9.
- [CrossRef] [PubMed] [Google Scholar]
- Parent perspectives of diabetes management in schools. Diabetes Educ. 2008;34:996-1003.
- [CrossRef] [PubMed] [Google Scholar]
- Improved diabetes management in Swedish schools: Results from two national surveys. Pediatr Diabetes. 2017;18:463-9.
- [CrossRef] [PubMed] [Google Scholar]






