Translate this page into:
Screening Sleep Abnormalities in Children with Prader–Willi Syndrome: Challenges in the Indian Scenario

*Corresponding author: Akanksha Chirag Parikh, Department of Pediatrics, Kokilaben Dhirubhai Ambani Hospital and Medical Research Institute, Mumbai, Maharashtra, India. gandhi.akanksha@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Parikh AC, Olety SS. Screening sleep abnormalities in children with Prader Willi syndrome: Challenges in the Indian scenario. J Pediatr Endocrinol Diabetes 2021;1:20-5.
Abstract
There is a high prevalence of sleep-related breathing disorders in the form of obstructive and central sleep apnea as well as spontaneous oxygen desaturation in children with Prader–Willi syndrome (PWS). Most cases are asymptomatic and if untreated go on to develop unfavorable neurodevelopmental, cardiovascular, and cerebrovascular outcomes. Hence, sleep study or polysomnography (PSG) is recommended in all children at the time of diagnosis as well as with the development of certain risk factors including symptoms of sleep apnea, before and after initiation of recombinant growth hormone (rGH) therapy. The use of rGH in children with PWS has been shown to improve central sleep apnea but also shown to be associated with worsening of OSA. PSG is ideally performed in a sleep laboratory. Various types of PSG devices are available depending on the biological parameters that are desired to be monitored. Sleep disorders in children are distinct from those seen in adults and have different diagnostic scoring criteria necessitating a trained pediatric sleep specialist to analyze the PSG recording. Through the clinical case vignette of a 14-year-old girl with PWS, severe obesity, and sleep disordered breathing, this review aims to highlight the need, timing, types, analysis, and interpretation of sleep studies in infants and older children with PWS, particularly in relation to rGH therapy. There is a paucity of literature on sleep studies in children with PWS in the local setting. Thus this review also suggests the need for adapting the existing Western guidelines for PSG in Indian children with PWS.
Keywords
Polysomnography
Sleep disordered breathing
Obstructive sleep apnea
Recombinant growth hormone therapy
Prader–Willi syndrome
INTRODUCTION
Sleep disordered breathing (SDB) is known to occur in almost 80% of children with Prader–Willi syndrome (PWS).[1] In addition to obesity, other factors such as hypotonia, anatomically small airways, and immature hypothalamic respiratory regulation contribute to the high prevalence of sleep apnea in this population.[2] Many comorbidities can result secondary to untreated SDB such as neurodevelopmental affection, cardiovascular disease, and stroke, necessitating its early detection and treatment.[3] Further, the use of recombinant growth hormone (rGH) therapy in children with PWS has raised questions regarding its safety in terms of worsening of obstructive sleep apnea (OSA) and sudden unexpected death.[4,5]
The existing guidelines pertaining to the conductance of polysomnography (PSG) in pediatric PWS patients are developed and suited for the Western population.[6,7] This review aims to answer some of the practical questions regarding the need, timing, types, analysis, and interpretation of sleep studies in infants and older children with PWS in the Indian setting, particularly in relation to rGH therapy.
CASE DETAILS
A 14-year-old girl, recently diagnosed as PWS by methylation studies, presented with history of excessive weight gain, particularly in the past 2 years. There was a history of increased calorie-rich food intake and sedentary lifestyle. She had cognitive impairment and went to a special school. She gave a history of snoring and gurgling sounds during sleep, but did not have nighttime arousals or excessive daytime sleepiness. On enquiry, the child gave no history suggestive of diabetes, raised intracranial tension, or slipped capital femoral epiphyses. Examination findings included a blood pressure of 110/70 mmHg, weight of 68.7 kg (1.89 SD), height of 128 cm (−4.0 SD), and BMI of 41 kg/m2 (4.5 SD). Typical features of PWS, such as almond shaped eyes, brachydactyly, abdominal obesity, and hypoplastic labia minora, were observed. Mild tonsillar hypertrophy was present. She was prepubertal with severe acanthosis and an unremarkable systemic examination. The child was advised certain blood investigations to look for metabolic syndrome and referred to the ear-nose-throat (ENT) clinic for the assessment of adenotonsillar hypertrophy. A PSG had been performed elsewhere to evaluate SDB.
Q1. How common is SDB in children with PWS?
The term “SDB” encompasses the various sleep-related respiratory conditions such as OSA, central apnea, and hypoventilation. Almost 80% of children with PWS suffer from SDB[1] in comparison to 1–13% of the general pediatric population.[8] In a recent study from South India, SDB was found to be present in 100% of children with PWS.[9] A high prevalence of SDB has been noted irrespective of age, sex, BMI centiles, ongoing growth hormone therapy, presence of symptoms of OSA, and the genotype, necessitating a look out for SDB in all children with PWS.[1]
Q2. Why are children with PWS at risk for SDB?
Children with PWS have a number of risk factors making them very prone for SDB. In older children, OSA has been attributed mainly to obesity, untreated hypothyroidism, restrictive lung capacity due to scoliosis, and physiological adenotonsillar hypertrophy (as seen in normal children between 2 and 10 years of age) which may be further worsened by concurrent rGH therapy.[1] On the other hand, OSA in infants occurs due to marked hypotonia causing airway collapse, respiratory muscle weakness, mid-facial hypoplasia, and sticky oral secretions.[2,10,11] Infants <2 years of age are equally susceptible to central sleep apnea due to hypothalamic and brainstem immaturity which usually improves with time.[12] It has been observed that unlike healthy individuals, one-third of children with PWS fail to respond to hypoxia and hypercapnia by increasing their minute ventilation. This blunted response leads to lower baseline blood oxygen saturation and higher carbon dioxide concentration in children with PWS.[3] Further, children with PWS often have nocturnal episodes of spontaneous oxygen desaturation due to altered REM sleep pattern, putting them at risk for narcolepsy as well.[13] Hence, a baseline sleep study has been recommended in all infants as well as children with PWS, particularly in the presence of certain risk factors as outlined in Box 1.[6]
|
The child’s investigations at presentation are shown in Table 1. Although fasting blood sugar was normal, the glycosylated hemoglobin was found to be in the pre-diabetic range. Rest of the metabolic and endocrine investigations were found to be normal. A radiograph of the nasopharynx revealed significant adenoid hypertrophy. An unattended level III overnight home sleep test had been performed using the four channel ApneaLink Air (version 2.0; ResMed, San Diego, CA, USA) polysomnogram device. Different parameters were recorded such as nasal flow, pulse, oxygen saturation, respiratory effort, and snoring. Adult respiratory criteria as shown in Table 2 were utilized by the particular device. The details and results of the PSG are depicted in Box 2 and Table 3.
| Parameter | Value |
|---|---|
| Fasting blood sugar (mg/dL) | 72 |
| HBA1c (%) | 6.1 |
| Total cholesterol (mg/dL) | 167.4 |
| Triglyceride (mg/dL) | 110.2 |
| HDL cholesterol (mg/dL) | 48 |
| LDL cholesterol (mg/dL) | 97.36 |
| AST (U/L) | 20.4 |
| ALT (U/L) | 18.9 |
| Blood urea (mg/dL) | 24.6 |
| Serum creatinine (mg/dL) | 0.87 |
| Serum Na/K/Cl (meq/L) | 138.6/4.5/103 |
| Serum calcium (mg/dL) | 10.2 |
| Serum phosphorus (mg/dL) | 5.3 |
| Serum alkaline phosphatase (IU/L) | 71 |
| Free T4 (ng/dL) | 1.2 |
| TSH (mIU/L) | 3.29 |
| 8 am serum cortisol (μg/dL) | 5.1 |
HbA1c: Glycosylated hemoglobin A1, HDL: High-density lipoprotein, LDL: Low-density lipoprotein, AST: Aspartate aminotransferase, ALT: Alanine aminotransferase, Na/K/Cl: Sodium/potassium/chloride, Free T4: Free thyroxine, TSH: Thyroid stimulating hormone
| Parameter | Adult | Pediatric |
|---|---|---|
| Apnea | Drop in airflow by ≥90% lasting for at least 10 s | Drop in airflow by ≥90% lasting for the duration as per OSA, CSA, mixed apnea criteria |
| OSA | Apnea with continued or increased respiratory effort throughout the event | Apnea for the duration of at least two breaths with respiratory effort throughout the event of absent airflow |
| CSA | Apnea with absence of inspiratory effort throughout the event | Apnea with absence of inspiratory effort throughout the event with
|
| Mixed sleep apnea | Apnea associated with absent respiratory effort in the initial portion of the event followed by resumption of the inspiratory effort in the second portion | Apnea lasting for the duration of at least two breaths and associated with absent respiratory effort during one portion and presence of inspiratory effort during another, regardless of which comes first |
| Hypopnea | Drop in airflow by ≥30% lasting for ≥10 s with an oxygen desaturation of ≥3% or an arousal | Drop in airflow by ≥30% lasting for at least two breath cycles with an oxygen desaturation of ≥3% or an arousal |
| Hypoventilation | Increase in arterial PaCO2>55 mmHg for ≥10 min | PaCO2 levels for > 25% of total sleep time > 50 mmHg |
OSA: Obstructive sleep apnea, CSA: Central sleep apnea, PaCO2: Partial pressure of carbon dioxide, AASM: American Academy of Sleep Medicine
| Parameter | Definition | Patient value | Interpretation | |
|---|---|---|---|---|
| OAHI | Obstructive apnea, mixed apnea and hypopnea events per hour during sleep | OAHI: 7/h OAI: 0.8/h HI: 6.3 |
Mild OSA (adult criteria) Moderate OSA (pediatric criteria) |
|
| Significant OAHI: Adult: Normal: OAHI < 5/h Mild: OAHI ≥ 5–15/h Moderate: OAHI ≧ 15–30/h Severe: OAHI > 30/h with symptoms of OSA such as unrefreshing sleep, daytime sleepiness, fatigue or insomnia, awakening with a gasping or choking sensation, loud snoring, or witnessed apnea |
Pediatric: Normal: OAHI < 1.5/h Mild: OAHI >1.5– < 5/h Moderate: OAHI > 5–<10/h Severe: OAHI >10/h |
|||
| CAI | Central apnea events per hour of sleep Significant CAI: >5 CAI events per hour during sleep |
0/h | Not significant | |
| ODI | ≥ 3% arterial desaturations in an hour of sleep Significant: >5 ODI events per hour |
21.4/h | Significant | |
OAHI: Obstructive apnea hypopnea index, OAI: obstructive apnea index, HI: Hypopnea index, CAI: Central apnea index, ODI: Oxygen desaturation index, OSA: Obstructive sleep apnea
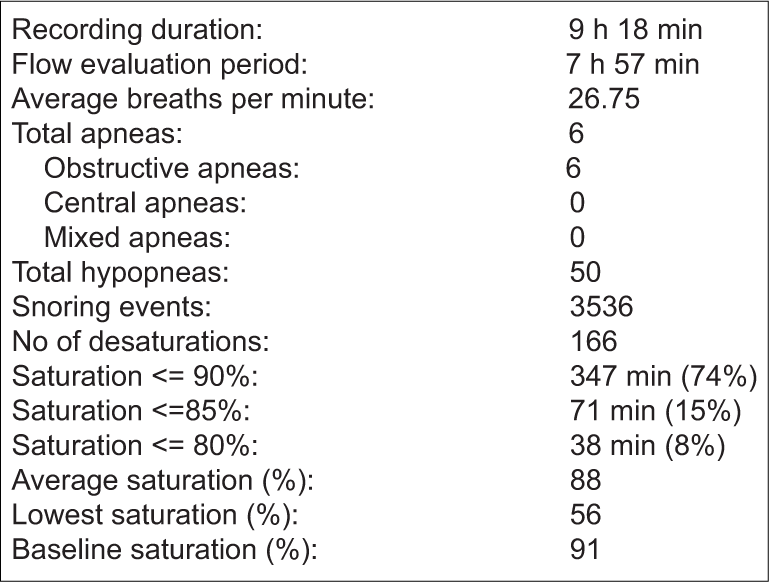
- Details of polysomnography recording of the patient.
Q3. What is a sleep study test and what is its use in children with PWS?
PSG or sleep study is a non-invasive test to monitor multiple biological parameters during sleep through electroencephalography (EEG), electrooculography (EOG), electrocardiography (ECG), electromyography (EMG), arterial oxygen saturation, respiratory effort, and snoring. Depending on the number of parameters measured, PSGs can be graded into four types as shown in Table 4 with type I being most extensive and type IV being the simplest.[14] The test is ideally performed in a sleep laboratory under the supervision of a sleep technician. However, in the event of unavailability of in-laboratory testing, an unattended sleep study may be conducted at home by portable monitoring.[15] The latter carries the disadvantage of interrupted recordings with restless children frequently detaching various channels sometimes requiring a repeat PSG. Sleep study is generally carried out on a routine week day and parents are advised to avoid late afternoon napping or unusually tiring activity on the day of the test.
| Type | Parameters assessed | Comment |
|---|---|---|
| I | EEG, EOG, EMG, ECG/pulse rate, airflow, respiratory effort, oxygen saturation, carbon dioxide concentration, blood pH | Most extensive form of PSG |
| II | EEG, EOG, EMG, ECG/pulse rate, airflow, respiratory effort, oxygen saturation | Measures minimum 7 channels |
| III | Airflow, respiratory effort, oxygen saturation, pulse rate or ECG | Measures minimum 4 channels |
| IV | Airflow, oxygen saturation | Measures 1 or 2 channels |
PSG: Polysomnography, EEG: Electroencephalography, EOG: Electrooculography, EMG: Electromyography, ECG: Electrocardiography
In children with PWS, a type III PSG may suffice to detect respiratory events such as apnea (obstructive, central, or mixed) and snoring and the physiological responses they elicit. However, in a child with suspected narcolepsy or sleep disorder, a type I PSG is required. A nasal cannula is applied to measure air flow and detect apnea and hypopnea. Along with this, an effort sensor attached to a Velcro belt is tied around the chest to sense respiratory effort; oxygen saturation is measured by a pulse oximeter. The measured data are logged into a recorder and analyzed subsequently by a trained pediatric sleep specialist.
Q4. How does one interpret a polysomnogram report?
The American Academy of Sleep Medicine (AASM) provides certain rules for the diagnosis of apnea and its classification in children and adults [Table 2].[16,17] It recommends the use of pediatric respiratory scoring criteria for all children less than or equal to 13 years of age; for older children up to 18 years, it allows the sleep clinician’s discretion over the choice of adult or pediatric criteria. Apnea refers to the cessation of airflow accompanied with or without respiratory effort. The number of apnea and hypopnea events per hour is reported as apnea-hypopnea index (AHI) [Table 3].[15,18,19] A separate index for obstructive and central sleep apnea events is usually reported. The main differences between adult and pediatric criteria for analysis of the PSG include duration of the respiratory event to diagnose apnea, definition of central apnea, and the severity scoring for OSA [Tables 2 and 3]. It is important to note the definitions employed by the sleep test device utilized for the recording and interpretation of the PSG as application of adult criteria in young children can underdiagnose OSA.
Children with PWS and OSA have been observed to have frequent dips in blood oxygen saturation levels.[3] Oxygen desaturation index (ODI) has been defined in children as the number of desaturations of more than or equal to 3%, per hour. Its value closely correlates with the severity of OSA.[10] However, in PWS, desaturations have also been seen to occur irrespective of the presence of OSA and may be attributed to the immaturity of respiratory centers in the hypothalamus.[20]
In our patient, being 14 years of age, adult criteria were used for analyzing the PSG. She had an AHI of 7 which would be diagnosed as mild OSA according to the adult criteria and moderate OSA with the pediatric scoring system. A low obstructive apneic index of 0.8 indicates that most sleep-related events were hypopneic. The child did not demonstrate central apnea but had a very severe desaturation index and a low baseline oxygen saturation.
Although our patient had mild to moderate OSA, in view of the severe obesity and significantly elevated desaturation index, treatment with rGH therapy was deferred. Lifestyle changes including adequate exercise, appropriate dietary measures, and food-related behavior therapy were advised. The ENT specialist recommended adenotonsillectomy (AT) which was refused by the patient’s family. The child was, therefore, advised the use of night time continuous positive pressure ventilation (CPAP). A decision to reconsider rGH therapy after at least 5–10% weight loss was taken.
Q5. What are the treatment options in children with PWS and SDB?
Akin to otherwise healthy children, initial steps to manage OSA include lifestyle changes with behavior modification. However, this has a limited effect in isolation, and other measures such as AT and positive pressure ventilation usually require to be undertaken.[3] Although, AT is considered to be an effective treatment of OSA in otherwise healthy children, it has been observed to be only partially effective in PWS. A meta-analysis by Lee et al. (2019) to assess the benefit of AT in children with PWS found that 71% of 41 children had a significant reduction in the severity of OSA albeit with residual mild disease.[21] In addition, common post-operative complications included hemorrhage, respiratory distress, and temporary velopharyngeal insufficiency. For children with residual OSA or those refusing surgery such as our patient, CPAP or bilevel positive airway pressure can be effectively tried.[10] In infants with central sleep apnea, low flow nasal oxygen at 0.25–1 liter/minute has been shown to lead to significant improvement in CAI and ODI.[12]
Q6. Is it necessary to perform a sleep study before rGH therapy?
In the year 2000, rGH was approved by the Food and Drug Administration for treatment in children with PWS. A baseline PSG before the initiation of rGH therapy can help detect undiagnosed OSA and has significant implications on treatment onset. A study by Vandeleur et al. revealed that 11 of the 15 children with PWS who were diagnosed with OSA on PSG before the onset of rGH therapy were previously asymptomatic.[20] Similar to our case, the decision to initiate rGH treatment was deferred in 80% of the patients till after SDB was addressed. Hence, it has been recommended for all children to undergo a PSG before initiation of rGH treatment.[6] Further, rGH therapy is contraindicated in individuals with PWS with severe obesity, uncontrolled diabetes, untreated severe sleep apnea, active cancer, and active psychosis.[7]
Q7. Should a sleep study be performed after starting rGH therapy?
Central sleep apnea is consistently seen to improve with rGH therapy in children with PWS.[22-24] On the other hand, the response of OSA is variable. A few studies have reported initial worsening of OSA in a some children as early as 6 weeks into therapy.[22,25]However, many of the children who had developed OSA were seen to have an upper or lower respiratory tract infection at the time of performing the PSG. A case report in 2011 described the close association of the development of OSA and rGH therapy in a young girl who developed OSA each time rGH therapy was initiated and had complete resolution of OSA both times when the therapy was withdrawn.[26] Another study by Berini et al. revealed a continued risk of development of OSA in some children even up till 4 years of rGH therapy.[24] In all such cases, the causal association of growth hormone and OSA could not be established due to the lack of a control group.
The dose of growth hormone may influence the development of adenotonsillar hypertrophy. Higher insulin-like growth factor-1 (IGF-1) levels have been observed in children with PWS with OSA compared to those without OSA.[3] Thus, 6–12 monthly monitoring of IGF-1 levels has been recommended with targeting the dose of rGH to keep the IGF-1 level between 0 and +2SD.[7]
The Growth Hormone Research Society consensus guidelines recommend performing a sleep study within 3–6 months of initiating therapy and earlier if the child develops symptoms of OSA.[7] They also recommend to avoid starting rGH therapy during an ongoing respiratory tract infection, however, if a child is on therapy and develops a upper respiratory tract infection (URTI), therapy need not be stopped. Instead, a close monitoring of symptoms such as snoring and increased daytime sleepiness along with the use of nighttime pulse oximetry may be a useful method to detect worsening of OSA and oxygen saturation levels warranting an early PSG.[5,7]
In summary, a high prevalence of SDB exists in children with PWS with majority being asymptomatic. Hence, a PSG is recommended for all children at the time of diagnosis and should be ideally performed in a sleep laboratory. In addition, PSG should be advised in children with severe obesity, symptoms of OSA, and before and a few months after the initiation of growth hormone therapy. The efficacy of interventions such as CPAP, AT, and nighttime oxygen therapy in reducing OSA can also be assessed by a repeat sleep study. The use of simple multi-night pulse oximetry, as a screening tool for a formal PSG, may be helpful but requires to be validated. There is a need for more studies from India regarding the feasibility of adapting existing Western guidelines for PSG in children with PWS.
Acknowledgments
We acknowledge the contributions made by Dr. Indu Khosla for her valuable insights into the practical aspects of sleep studies in children in the local population.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Prader Willi syndrome and obstructive sleep apnea: Co-occurrence in the pediatric population. J Clin Sleep Med. 2014;10:403-9.
- [CrossRef] [PubMed] [Google Scholar]
- Respiratory control in children with Prader-Willi syndrome. Eur J Pediatr. 1996;156:65-8.
- [CrossRef] [PubMed] [Google Scholar]
- Disorders of sleep and ventilatory control in Prader-Willi syndrome. Diseases. 2016;4:23.
- [CrossRef] [PubMed] [Google Scholar]
- Cause of sudden, unexpected death of Prader-Willi syndrome patients with or without growth hormone treatment. Am J Med Genet A. 2005;136:45-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prader-Willi syndrome and growth hormone therapy: Take a deep breath and weigh the data. J Pediatr. 2013;162:224-6.
- [CrossRef] [PubMed] [Google Scholar]
- Available from: https://www.pwsausa.org/wp-content/uploads/2016/01/medicalalertbooklet.pdf [Last accessed on 2015 Jun 04]
- Growth Hormone Research Society workshop summary: Consensus guidelines for recombinant human growth hormone therapy in Prader-Willi syndrome. J Clin Endocrinol Metab. 2013;98:E1072-87.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:242-52.
- [CrossRef] [PubMed] [Google Scholar]
- A comparitive study of polysomnography findings in children with Prader Willi syndrome and nonsyndromic children. Indian J Sleep Med. 2020;15:65-8.
- [CrossRef] [Google Scholar]
- Sleep disordered breathing in patients with Prader-Willi syndrome: A multicenter study. Pediatr Pulmonol. 2015;50:1354-9.
- [CrossRef] [PubMed] [Google Scholar]
- The upper airway and sleep apnoea in the PraderWilli syndrome. Clin Otolaryngol Allied Sci. 1994;19:193-7.
- [CrossRef] [PubMed] [Google Scholar]
- Clinically important age-related differences in sleep related disordered breathing in infants and children with Prader-Willi syndrome. PLoS One. 2014;9:e101012.
- [CrossRef] [PubMed] [Google Scholar]
- The Relationship between depressive symptoms and obstructive sleep apnea in pediatric populations: A meta-analysis. J Clin Sleep Med. 2013;9:1213-20.
- [CrossRef] [PubMed] [Google Scholar]
- Polysomnography test and sleep disordered breathing in Prader-Willi syndrome. Mater Plast. 2014;51:331-5.
- [Google Scholar]
- Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:e714-55.
- [CrossRef] [PubMed] [Google Scholar]
- Rules for scoring respiratory events in sleep: Update of the 2007 AASM manual for the scoring of sleep and associated events: Deliberations of the sleep apnea definitions task force of the American academy of sleep medicine. J Clin Sleep Med. 2012;8:597-619.
- [CrossRef] [PubMed] [Google Scholar]
- The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications (1st ed). Westchester, IL: American Academy of Sleep Medicine; 2007.
- [Google Scholar]
- Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. The report of an American academy of sleep medicine task force. Sleep. 1999;22:662-89.
- [CrossRef] [PubMed] [Google Scholar]
- Are sleep studies helpful in children with Prader-Willi syndrome prior to commencement of growth hormone therapy? J Paediatr Child Health. 2013;49:238-41.
- [CrossRef] [PubMed] [Google Scholar]
- Adenotonsillectomy for the treatment of obstructive sleep apnea in children with Prader-Willi syndrome: A meta-analysis. Otolaryngol Head Neck Surg. 2020;162:168-76.
- [CrossRef] [PubMed] [Google Scholar]
- Short-term effects of growth hormone on sleep abnormalities in Prader-Willi syndrome. J Clin Endocrinol Metab. 2006;91:413-7.
- [CrossRef] [PubMed] [Google Scholar]
- Sleep-related breathing disorders in prepubertal children with Prader-Willi syndrome and effects of growth hormone treatment. J Clin Endocrinol Metab. 2006;91:4911-5.
- [CrossRef] [PubMed] [Google Scholar]
- Growth hormone therapy and respiratory disorders: Long-term follow-up in PWS children. J Clin Endocrinol Metab. 2013;98:E1516-23.
- [CrossRef] [PubMed] [Google Scholar]
- Sleep-disordered breathing in children with PraderWilli syndrome in relation to growth hormone therapy onset. Horm Res Paediatr. 2020;93:85-93.
- [CrossRef] [PubMed] [Google Scholar]
- Longitudinal association between growth hormone therapy and obstructive sleep apnea in a child with Prader-Willi syndrome. J Clin Endocrinol Metab. 2011;96:29-33.
- [CrossRef] [PubMed] [Google Scholar]






