Translate this page into:
Pituitary stalk interruption with multiple pituitary hormone deficiencies associated with a roundabout guidance receptor 1 gene mutation

*Corresponding author: Shamkiran, Department of Paediatric and Adolescent Endocrinology, SPARSH Hospital, Bengaluru, Karnataka, India. skfeb1593@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Shamkiran, Sheshadri T, Anil MU. Pituitary stalk interruption with multiple pituitary hormone deficiencies associated with a roundabout guidance receptor 1 gene mutation. J Pediatr Endocrinol Diabetes. 2024;4:139-42. doi: 10.25259/JPED_41_2024
Abstract
Pituitary stalk interruption syndrome (PSIS) is a rare condition characterized by multiple pituitary hormone deficiencies and a triad of distinctive features on imaging. We report a case of a 2.3-year-old male child who presented with hyponatremic seizures and was subsequently diagnosed with PSIS, identified to have a heterozygous mutation in the roundabout guidance receptor 1 (ROBO1) gene through clinical exome sequencing. This report emphasizes the importance of imaging in cases of hyponatremic seizures and represents the second documented case from India of classical PSIS associated with a novel ROBO1 gene mutation.
Keywords
Multiple pituitary hormone deficiency
Pituitary stalk interruption syndrome
Roundabout guidance receptor 1 (ROBO1) gene
INTRODUCTION
Pituitary stalk interruption syndrome (PSIS) is distinguished by multiple pituitary hormone (MPH) deficiencies and is identified on magnetic resonance imaging (MRI) by a triad consisting of aplastic or hypoplastic anterior pituitary, an ectopic posterior pituitary, and interrupted pituitary stalk.[1,2] This rare disorder has an incidence of 0.5/100,000 births.[3,4] Although the cause remains unknown in most the of cases, mutations in certain genes, such as HESX1, SOX3, OTX2, PROKR2, LHX4, CDON, and GPR161, have been linked with PSIS. The roundabout guidance receptor 1 (ROBO1) gene mutation has been documented in ten patients so far, including one from India.[5] Here, we report second documented case from India of classical PSIS associated with a novel ROBO1 gene mutation.
CASE REPORT
A 2-year, 3-month-old male child 2nd born to non-consanguineous marriage [Figure 1] presented with fever, vomiting, and one episode of generalized tonic clonic seizures (GTCS). At presentation, the child was drowsy but arousable, with a Glasgow Coma Scale (GCS) score of 10/15. He was admitted to the pediatric intensive care unit (PICU) for further treatment. Routine investigations were sent, which revealed a negative sepsis screen, hyponatremia with a sodium level of 110 mmoL/L, normal potassium, and renal functions [Table 1]. The child was started on symptomatic treatment with IV fluids, Inj. Levetiracetam, and IV antibiotics (Inj. Ceftriaxone).
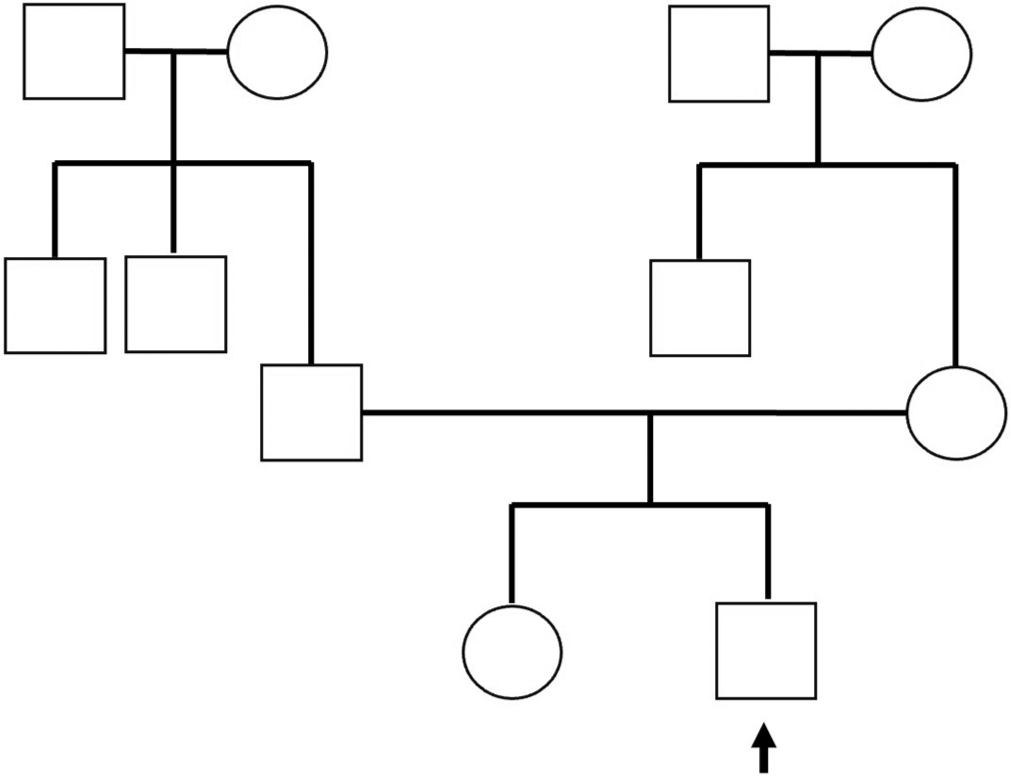
- Three generation pedigree chart (arrow mark-proband under examination).
| Parameter | Results | Normal values |
|---|---|---|
| Hemoglobin | 9.7 g/dL | 11–14 g/dL |
| Total white blood counts | 3460 cells/cumm | 5000–15000 cells/cumm |
| Differential leukocyte counts | N 35.8%, L 57.8% | |
| Total platelets | 1.7 lakhs/cumm | 1.5–4.5 lakhs/cumm |
| Serum C-reactive protein | <6 mg/dL | <6 mg/dL |
| Serum sodium | 110 mmoL/L | 135–145 mmoL/L |
| Serum potassium | 4.6 mmoL/L | 3.5–5.5 mmoL/L |
| Serum chloride | 76 mmoL/L | 98–107 mmol/L |
| Serum urea | 8 mg/dL | 10–38 mg/dL |
| Serum creatinine | 0.3 | 0.5–1.0 mg/dL |
| Serum osmolality | 229 mOsm/Kg | 275–300 mOsm/Kg |
| Urine sodium | 108 mmoL/L | 30–90 mmol/L |
| Urine potassium | 17.9 mmoL/L | 0–20 mmol/L |
| Urine chloride | 112 mmoL/L | 27–331 mmol/L |
| Urine osmolality | 319 mOsm/Kg | 500–1200 mOsm/kg |
| Serum uric acid | 2.7 mg/dL | 2.4–5.4 mg/dL |
| Random blood glucose | 81 mg/dL | 70–180 mg/dL |
| 8 am serum cortisol | 0.2 µg/dL | 3–21 µg/dL |
| Plasma ACTH | 16.11 pg/mL | 0–46 pg/mL |
| Serum free T4 | 0.37 ng/dL | 0.96–1.77 ng/dL |
| Serum Total T4 | 3.25 µg/dL | 5–18.5 µg/dL |
| Serum TSH | 1.55 µIU/mL | 0.7–4.4 µIU/mL |
| Serum prolactin | 14.7 ng/mL | 3.7–17.9 ng/mL |
| Serum IGF-1 levels | <15 ng/mL | 51–303 ng/mL |
ACTH: Adrenocorticotropic hormone, TSH: Thyroid-stimulating hormone, IGF-1: Insulin-like growth factor 1
On physical examination, he had short stature with a height of 80 cm (−2.9 Z score), MPH of 167 cm (−0.68 Z score), and weight of 10 kg (−2.09 Z). There were no midfacial hypoplasia or dysmorphic features. On further evaluation, serum cortisol was low 0.9 μg/dL; adrenocorticotropic hormone (ACTH) was 16.11 pg/mL; free T4 (FT4) and total T4 (TT4) were low with inappropriately normal thyroid-stimulating hormone (TSH) suggestive of central hypothyroidism, and serum insulin-like growth factor 1 (IGF-1) was <15 ng/mL. Serum osmolality was low, with urine osmolality >100 mOsm/kg and raised urine sodium. Hence, fluid restriction and sodium correction with hypertonic saline (3% normal saline) bolus 5 mL/kg followed by continuous infusion to correct sodium level by 12 meq/day was started. A screening MRI brain revealed an ectopic posterior pituitary bright spot near the floor of the third ventricle, as well as a small anterior pituitary gland with no visible pituitary stalk, indicating PSIS [Figure 2]. In view of MRI findings, hypopituitarism and inadequate response to fluid restriction and sodium correction, hydrocortisone replacement at 10 mg/m2/day was initiated, and serum sodium levels improved, considerably as shown in Figure 3. Thyroxine supplementation at 25 μg/day commenced 48 h following steroid replacement (thyroxine will increase the clearance of cortisol precipitating the adrenal deficiency; hence, cortisol should be replaced before starting thyroxine in cases of hypopituitarism). His urine output and serum electrolytes were normal following hydrocortisone and thyroxine supplementation. Parents were counseled in detail regarding PSIS and the requirement for hormone replacement therapy. They were also advised about the need for a stress dose of steroids during illness or surgery.
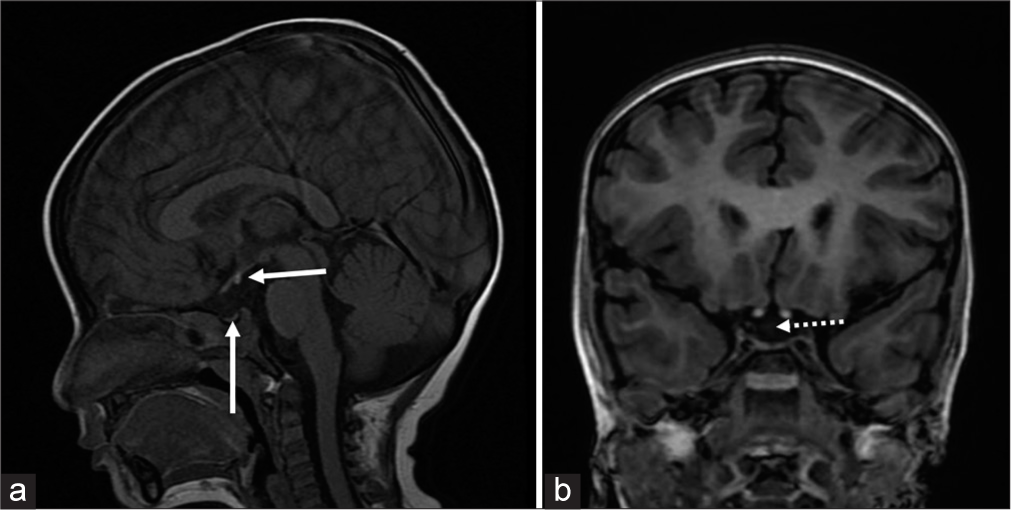
- (a) A 2.3-year-old male with pituitary stalk interruption syndrome presented with hyponatremic seizures. T1 fast spin echo sagittal view of magnetic resonance imaging (MRI) brain shows small anterior pituitary (vertical white arrow) and ectopic posterior pituitary (horizontal white arrow) and (b) 3D-spoiled gradient echo of coronal view of MRI brain shows absent pituitary stalk (white dotted arrow).
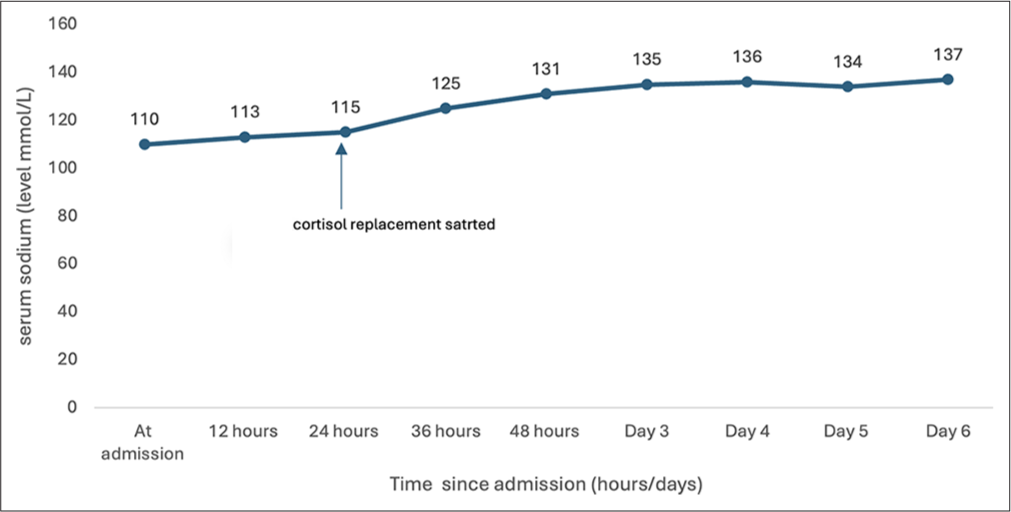
- Graph showing the trend in change of serum sodium from admission till discharge.
At 6 months, he had poor growth velocity (no height increment), so a growth hormone (GH) stimulation test was performed, which revealed a peak GH response of 1.09 ng/mL, indicating GH deficiency (GHD), and weekly GH injections at 0.44 mg/kg/week was started. A clinical exome sequencing was sent and it revealed a heterozygous missense variant (c.1904G>T) (p.Arg635Met) in the ROBO1 gene, confirming the diagnosis of PSIS. Ophthalmology review is planned on a follow-up.
DISCUSSION
Our patient had MPH deficiencies (MPHD) in addition to the classical triad of PSIS. Interestingly, the child presented with hyponatremic seizures, which is not unusual as few case studies have highlighted hyponatremic seizures as an early presentation in PSIS.[6,7]
In our patient, hyponatremia is explained by secondary adrenal insufficiency, which is caused by hypothalamic-pituitary-adrenal axis dysfunction. Cortisol normally has a negative feedback loop with both corticotropin-releasing hormone (CRH) and ACTH. Cortisol insufficiency causes increased hypothalamic production of CRH, which acts as an extra ADH secretagogue. Reduced ACTH and cortisol production occurs when there is a defect in the anterior pituitary or in the pituitary-hypothalamus communication pathway, as in PSIS. However, low cortisol levels stimulate the production of CRH, which serves as ADH secretagogue. Moreover, cortisol suppresses the release of ADH but in cases of adrenal insufficiency, this inhibition is lost leading to further increase in ADH and hyponatremia. Therefore, when a patient develops hyponatremia, he/she exhibits no characteristic syndrome of inappropriate antidiuretic hormone secretion (SIADH) behavior, and responds promptly to cortisol replacement. In such scenarios, an MRI of brain becomes crucial for diagnosis.
Our patient developed MPHD secondary to PSIS as a result of a heterozygous missense variant in the ROBO1 gene (c.1904G>T) (p.Arg635 Met). In 2017, Bashamboo et al.[8] described the first five cases of novel ROBO1 gene mutation. Three of these five patients had isolated GHD, whereas the other two had MPHD along with ocular abnormalities such as ptosis and strabismus.
The first case report from India by Misgar et al.[5] found a heterozygous frameshift mutation in the ROBO1 gene in a 2.9-year-old boy with MPHD with the classical PSIS triad. Another case from China, described by Liu and Chen,[9] was a 4-year-old boy with hyponatremia and convulsions. He was eventually diagnosed with MPHD due to PSIS, and similar to our patient, he had a heterozygous missense mutation in ROBO1.
The ROBO1 is a member of a gene family that produces proteins involved in axon guidance and cell migration, specifically in the central nervous system. It interacts with its ligand Slit, directing axons to the desired locations. Slit/Robo signaling promotes axonal elongation and branching in sensory neurons, cortical cells, and dendritic cells, hence actively participating in neuronal extension and branching.[9] Mutations in the ROBO/Slit signaling system can cause abnormalities in hippocampal and callosal commissure projections, thalamocortical and corticothalamic projections, and optic chiasm development in animals.[10] In the developing human brain, the lack of ROBO1 expression can cause ectopic differentiation of forebrain neurons.[10] There are case reports mentioning association of ROBO1 mutation with neurodevelopmental disorders, facial dysmorphism, and optic pathway abnormalities.[5,6,8] Our patient had no ophthalmological involvement at present, but a periodic follow-up with ophthalmologist is advised.
The specific mechanism by which disruption of ROBO1 signaling causes PSIS is uncertain; however, it could involve the Notch effector gene Hes1.[7] Thus, more clinical studies are needed to investigate the roles of the ROBO1 gene and its impact on pituitary development.
CONCLUSION
Our patient presented with hyponatremic seizures, and on further examination, he turned out to have MPHD due to PSIS; hyponatremic seizures were most likely caused by secondary adrenal insufficiency. A strong index of suspicion, as well as a brain MRI, are required for diagnosis in such scenario. Our patient’s PSIS is caused by a heterozygous missense variation in the ROBO1 gene. Similar cases have revealed unique ROBO1 mutations connected to MPHD and ocular abnormalities; however, the precise mechanism involving ROBO1 in PSIS is unknown, necessitating further clinical research.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Anterior and posterior lobes of the pituitary gland: Assessment by 1.5 T MR imaging. J Comput Assist Tomogr. 1987;11:214-20.
- [CrossRef] [PubMed] [Google Scholar]
- Magnetic resonance imaging in the diagnosis of growth hormone deficiency. J Pediatr. 1992;120:886-91.
- [CrossRef] [PubMed] [Google Scholar]
- 17q21.31 microdeletion in a patient with pituitary stalk interruption syndrome. Eur J Med Genet. 2011;54:369-73.
- [CrossRef] [PubMed] [Google Scholar]
- Congenital adenohypophysis aplasia: Clinical features and analysis of the transcriptional factors for embryonic pituitary development. J Endocrinol Invest. 2006;29:208-13.
- [CrossRef] [PubMed] [Google Scholar]
- Pituitary stalk interruption syndrome due to novel ROBO1 mutation presenting as combined pituitary hormone deficiency and central diabetes insipidus. J Pediatr Endocrinol Metab. 2024;7:477-81.
- [CrossRef] [PubMed] [Google Scholar]
- A case of pituitary stalk interruption syndrome with intermittent seizures as the first presentation. Neuro Endocrinol Lett. 2016;37:469-72.
- [Google Scholar]
- Diagnosis of endocrine disease: Pituitary stalk interruption syndrome: Etiology and clinical manifestations. Eur J Endocrinol. 2019;181:199-209.
- [CrossRef] [PubMed] [Google Scholar]
- Mutations in the human ROBO1 gene in pituitary stalk interruption syndrome. J Clin Endocrinol Metab. 2017;102:2401-6.
- [CrossRef] [PubMed] [Google Scholar]
- A novel missense mutation in human receptor roundabout-1 (ROBO1) gene associated with pituitary stalk interruption syndrome. J Clin Res Pediatr Endocrinol. 2020;12:212-7.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular mechanisms of pituitary organogenesis: In search of novel regulatory genes. Mol Cell Endocrinol. 2010;323:4-19.
- [CrossRef] [PubMed] [Google Scholar]






