Translate this page into:
Patterns of incidence and characteristics of youth with new-onset diabetes mellitus during the COVID era

*Corresponding author: Emily L. Montgomery, Department of Pediatric Endocrinology, University of Louisville, Louisville, United States. emily.montgomery.1@louisville.edu
-
Received: ,
Accepted: ,
How to cite this article: Montgomery EL, Jawad K, Wintergerst K, Watson S. Patterns of incidence and characteristics of youth with new-onset diabetes mellitus during the COVID era. J Pediatr Endocrinol Diabetes. 2024;4:70-8. doi: 10.25259/JPED_5_2024
Abstract
Objectives:
The incidence of diabetes mellitus (DM) in children, adolescents, and young adults has been on the rise for many decades. The COVID-19 pandemic has been associated with a dramatic increase in new cases of pediatric DM and a shift in the seasonal pattern of incidence. We aimed to determine the incidence of DM and its subtypes during the COVID-19 pandemic (2020–2022) and pre-pandemic (2017–2019). We sought to assess for a difference in seasonality and temporal pattern of new-onset DM between the two periods and aimed to describe and compare the clinical characteristics of the patients diagnosed during this time.
Material and Methods:
In this retrospective chart review, data were collected from medical records for all patients aged 1–21 years diagnosed with DM at our center between January 1, 2017 and December 31, 2022.
Results:
The incidence of DM at our center increased 38% during the pandemic (incidence rate ratio [IRR] 1.38, 95% confidence interval; [CI] 1.20–1.58). The incidence of type 1 diabetes (T1D) increased 11% (IRR 1.11, 95% CI 1.01–1.23) and the median body mass index percentile increased for those diagnosed during the pandemic (P = 0.012). The incidence of type 2 diabetes (T2D) increased 238% (IRR 3.38, 95% CI 2.17–5.28) during the pandemic, with the highest rate of diagnosis in a younger age group (P = 0.015). The pattern of incidence of T1D shifted from lowest in the summer pre-pandemic to highest in the summer during the pandemic (IRR 1.82, 95% CI 1.22–2.72). The overall incidence trend for DM, T1D, and T2D, peaked in spring 2021 and then declined until it stabilized in summer 2022.
Conclusion:
Our study showed a persistent increase in the incidence of both T1D and T2D in the pediatric population during the pandemic. Those diagnosed with T2D during the pandemic were younger than those diagnosed in the pre-pandemic period. There was a shift in seasonal pattern of T1D incidence during the pandemic with the highest rates of incidence during the summer. Further studies are needed to evaluate the underlying mechanisms of the persistent increase in incidence.
Keywords
Type 1 diabetes
Type 2 diabetes
Pediatric
Adolescent
COVID
INTRODUCTION
The incidence of diabetes mellitus (DM) in children, adolescents, and young adults has been on the rise for many decades.[1-4] For type 1 diabetes (T1D), the incidence in the USA has been increasing by 2% per year and has followed a seasonal pattern, with the highest rate of new diagnoses in the wintertime and the lowest in the summertime.[3-5] The incidence of type 2 diabetes (T2D) has been increasing by 5.3% per year, with the peak rate of incidence occurring in the late summer.[3] The COVID-19 pandemic has been associated with a dramatic increase in new cases of DM[1,5-23] as well as concern for an increased severity of disease at presentation.[6-10,12,14-16,19,21-29]
During the first wave of the COVID-19 pandemic, it was unclear whether there was a true universal increase in the incidence of DM in pediatric patients, with conflicting reports from different countries.[18,19,21,24,26,28,30] However, more recent publications have reviewed the incidence through the end of 2021 and determined that while the initial shutdown period had a decrease in incidence in most regions where it was reported, the incidence of DM, both T1D and T2D, subsequently increased and has not returned to pre-pandemic rates.[1,5-20,31] The degree of severity at the time of presentation with new-onset DM has not been universally reported, with some centers seeing an increase in severity and others showing no change compared to the pre-pandemic rates of diabetic ketoacidosis (DKA) and hyperosmolar hyperglycemic syndrome (HHS).[6-12,14-16,18-25,28-33]
Whether the sustained increase in T1D incidence has exceeded the predicted rate based on historical rates of annual increase in DM remains up for debate.[1,5,8,12,13,17] However, the change in the seasonal pattern of incidence was notably consistent throughout Europe and North America in 2020, with the highest rates of new-onset T1D diagnosed in the summer and early fall.[1,5,6,8,9,11,13,17] It was noted that in 2021, the shift in the seasonal pattern of T1D incidence continued in the USA/North America; however, most of the European groups saw a return to the pre-pandemic seasonal pattern of T1D incidence.[5,13] To the best of our knowledge, these data have not been explored beyond early 2022.
There are fewer studies regarding the incidence of T2D in children and adolescents, both pre-pandemic and during the pandemic, but of those available, there has been a general uptrend in annual incidence, with a more marked increase during 2020 and 2021.[2,3,7,9,16,17,19,20,23,24] The timing of diagnosis shifted from the pre-pandemic peak during August and later summer to a steady increase throughout the pandemic from spring 2020 to early 2021.[9,16,17,23]
As described above, there has been significant interest and publication regarding the patterns of incidence and characteristics of patients with new-onset DM during the pandemic compared to the years before the pandemic. At this time, the majority of available data has been limited to 2020–2021, and minimal to no data has addressed the pattern of incidence through the end of 2022 in both T1D and T2D. In this retrospective chart review, we aimed to determine the incidence of DM and its subtypes at our center, Wendy Novak Diabetes Institute, during the three years pre-COVID-19 pandemic (2017–2019) and the pandemic (2020–2022). We sought to assess the seasonality and temporal pattern of new-onset DM during the pandemic compared to the 3 years prior. We additionally aimed to describe and compare the clinical characteristics of the patients diagnosed during the pandemic with those diagnosed before the pandemic.
MATERIAL AND METHODS
This retrospective chart review included all patients aged 12 months to 21 years diagnosed with DM at the Wendy Novak Diabetes Institute or Norton Children’s Hospital in Louisville, Kentucky, between January 1, 2017, and December 31, 2022. This facility is a tertiary referral center and provides care to the majority of pediatric patients with DM in this region. The data were dichotomized by year of diagnosis, with 2017–2019 considered pre-pandemic and 2020–2022 considered pandemic. The study was approved by the Institutional Review Board at the University of Louisville.
Excluded from this study were individuals who did not meet the American Diabetes Association diagnostic criteria,[34] those with DM secondary to another medical condition, medication-induced DM, a diagnosis of maturity-onset diabetes in the young, and those who did not receive their new-onset DM care at our facility. Data for patients who were diagnosed with DM, T1D, or T2D during the time period listed above were collected from their records at diagnosis and first follow-up visit.
Demographic and clinical information were collected from patient medical records in the electronic medical record system. DM type was determined by the presence or absence of autoantibodies and insulin resistance at diagnosis. Patients who were positive for at least one autoantibody (GAD65, Islet antigen 2, and insulin) were classified as T1D. Those without positive autoantibodies who did not have evidence of insulin resistance were also classified as T1D. Those who had evidence of insulin resistance on physical examination without positive autoantibodies were classified as T2D. DKA, HHS, and mixed DKA-HHS were defined per the most recent International Society for Pediatric and Adolescent Diabetes clinical consensus guidelines published in 2022.[35]
Statistical analysis
Within the patient characteristics, continuous variables were analyzed using the Wilcoxon Rank-Sums test. Chi-square and Fisher’s exact tests were used to compare categorical variables of patient characteristics between the two periods. Kruskal-Wallis and Fisher’s exact tests were used to compare patient characteristics for 2020, 2021, and 2022; P < 0.05 was significant. To examine an association between severity at presentation with the pre-pandemic and pandemic periods, generalized multinomial logistic regression analysis was used.
The population of people 1–21 years of age in the regions where our patients live was calculated using the census tract data. The incidence rate was determined using this population of people aged 1–21 years of age between 2017 and 2022. The rate is per 100,000 children. A time series analysis was performed to evaluate the trend of DM incidence before the pandemic and during the pandemic. The incidence rate was decomposed into seasonality, trend, and random error using the Decompose function within the R software. Seasons were designated as follows: winter (December–February), spring (March–May), summer (June–August), and fall (September–November). Poisson regression models were used to determine the relationship between incidence and season. The cases were dichotomized into pre-pandemic (January 1, 2017–February 29, 2020) and pandemic (March 1, 2020–December 31, 2022) to assess for significant differences between these periods for all new diagnoses of DM as well as the subtypes, T1D and T2D. In addition, the pandemic years were compared (2020 vs. 2021–2022 vs. 2021) to determine changes in the incidence trend within the 3 years. Poisson regression models were used to evaluate differences in the monthly incidence rate between the pre-pandemic and pandemic periods and within the pandemic period. The SCALE-PEARSON model was used to account for overdispersion. Statistical analysis was performed using SAS software version 9.4 (SAS Institute, Cary, NC).
RESULTS
Of the 1139 charts reviewed, 969 patients met the inclusion criteria and had sufficient data for analysis (763 T1D and 206 T2D). Patient demographics and characteristics are shown in Tables 1 and 2.
| Characteristic | Pre-pandemic | Pandemic | Univariate analysis |
|---|---|---|---|
| 2017–2019 | 2020–2022 | P-value | |
| Total, n(%) | 361 (47.3) | 402 (52.7) | |
| Sex, n(%) | 0.554a | ||
| Female | 162 (44.9) | 189 (47.0) | |
| Male | 199 (55.1) | 213 (53.0) | |
| Race, n(%) | 0.215a | ||
| non-Hispanic White | 289 (80.1) | 327 (81.3) | |
| non-Hispanic Black | 41 (11.4) | 41 (10.2) | |
| Hispanic | 8 (2.2) | 7 (1.7) | |
| Other | 7 (1.9) | 1 (0.2) | |
| Two or more races | 14 (3.9) | 18 (4.5) | |
| Age group, n(%) | 0.685a | ||
| 0–7 | 87 (24.1) | 110 (27.4) | |
| 7–9 | 51 (14.1) | 49 (12.2) | |
| 9–11 | 64 (17.7) | 60 (14.9) | |
| 11–13 | 54 (15.0) | 58 (14.4) | |
| 13–15 | 50 (13.9) | 51 (12.7) | |
| 15–17 | 34 (9.4) | 42 (10.4) | |
| 17–21 | 21 (5.8) | 32 (8.0) | |
| C–peptide (ng/mL), Median (Q1–Q3) | 0.6 (0.3−0.9) | 0.5 (0.3−1.0) | 0.765b |
| BMI percentile, Median (Q1–Q3) | 53.4 (17.9−89.0) | 67.1 (32.1−91.8) | 0.012b |
| Household incomed, Median (Q1–Q3) | $64,283 | $66,062 | 0.618b |
| ($46,781−$83,333) | ($46,620−$85,498) | ||
| SVI, Median (Q1–Q3) | 0.5 (0.3 – 0.7) | 0.4 (0.3 – 0.7) | 0.489b |
| Education level percentd, Median (Q1–Q3) | |||
| High school or less | 47.9 (34.5−57.9) | 48.8 (34.5−59.0) | 0.656b |
| Some colleges or more | 52.2 (42.1−65.5) | 51.2 (41.0−65.5) | 0.656b |
| 9th grade | 3.4 (1.6−5.8) | 3.4 (1.8−5.8) | 0.650b |
| 9–12th grade, no diploma | 7.4 (4.3−10.8) | 7.5 (4.4−10.4) | 0.976b |
| High school graduate | 35.5 (27.1−41.2) | 36.1 (26.6−41.6) | 0.688b |
| In some college, no degree | 21.8 (18.2−25.6) | 21.1 (18.2−24.5) | 0.122b |
| Associate degree | 8.6 (6.8−10.3) | 8.4 (6.7−9.9) | 0.427b |
| Bachelor degree | 11.6 (7.9−18.4) | 11.1 (7.9−20.3) | 0.930b |
| Graduate or professional degree | 7.1 (4.4−12.2) | 7.6 (4.8−12.6) | 0.459b |
aP-value of Chi-square test, bP-value of Wilcoxon Rank-Sum test, dU.S. Census Bureau (2017) by census tract level. All continuous variables were non-normal and were described using the median (interquartile range). SVI:Social vulnerability index, BMI: Body mass index, T1D: Type 1 diabetes mellitus. Bold text signifies statistical significance of P-value of <0.05.
| Characteristic | Pre-pandemic | Pandemic | Univariate analysis |
|---|---|---|---|
| 2017–2019 | 2020–2022 | P-value | |
| Total, n(%) | 47 (22.8) | 159 (77.2) | |
| Sex, n(%) | |||
| Female | 24 (51.1) | 79 (49.7) | 0.868a |
| Male | 23 (48.9) | 80 (50.3) | |
| Race, n(%) | |||
| non-Hispanic White | 19 (40.4) | 41 (25.8) | 0.397b |
| non-Hispanic Black | 19 (40.4) | 75 (47.2) | |
| Hispanic | 5 (10.6) | 24 (15.1) | |
| Other | 0 (0.0) | 2 (1.3) | |
| Two or more races | 4 (8.5) | 10 (6.3) | |
| Age group (years), n(%) | |||
| 0–7 | 0 (0.0) | 1 (0.6) | 0.015b |
| 7–9 | 1 (2.1) | 2 (1.3) | |
| 9–11 | 2 (4.3) | 14 (8.8) | |
| 11–13 | 8 (17.0) | 43 (27.0) | |
| 13–15 | 7 (14.9) | 40 (25.2) | |
| 15–17 | 24 (51.1) | 35 (22.0) | |
| 17–21 | 5 (10.6) | 24 (15.1) | |
| C–peptide (ng/mL), Median (Q1–Q3) | 3.8 (2.6–6.6) | 4.3 (2.5–6.8) | 0.947c |
| BMI percentile, Median (Q1–Q3) | 99.4 (98.5–99.7) | 99.3 (98.6–99.6) | 0.930c |
| Household incomed, Median (Q1–Q3) | $49,688 | $47,110 | 0.924c |
| ($35,345–$60,068) | ($34,476–$64,117) | ||
| SVI, Median (Q1–Q3) | 0.7 (0.5–0.8) | 0.6 (0.4–0.9) | 0.771c |
| Education level percentd, Median (Q1–Q3) | |||
| High school or less | 54.0 (50.0−59.3) | 53.2 (43.1−59.5) | 0.313 c |
| Some college or more | 46.0 (40.7−50.0) | 46.8 (40.5−56.9) | 0.313 c |
| Less than 9th grade | 5.4 (4.3–7.9) | 4.6 (2.5–6.2) | 0.017c |
| High school, no diploma | 10.1 (7.9–13.1) | 8.9 (6.5–13.1) | 0.262c |
| High school graduate or GED | 37.6 (30.1–41.2) | 36.0 (31.3–40.5) | 0.656c |
| Some college, no degree | 23.4 (19.8–24.9) | 22.5 (19.7–27.0) | 0.957c |
| Associate degree | 7.9 (6.3–9.7) | 8.3 (6.3–10.5) | 0.340c |
| Bachelor degree | 7.9 (5.8–11.7) | 9.3 (5.9–13.8) | 0.349c |
| Graduate/Professional | 4.9 (3.3–7.7) | 5.8 (2.9–8.5) | 0.333c |
aP-value of Chi-square test, bP-value of Fisher’s Exact test, cP-value of Wilcoxon Rank-Sum test, dU.S. Census Bureau (2017) by census tract level. All continuous variables were non-normal and were described using the median (interquartile range). SVI: Social vulnerability index, BMI: Body mass index, GED: General educational development, T2D: Type 2 diabetes mellitus. Bold text signifies statistical significance of P-value of <0.05.
The incidence of DM was higher during the pandemic period, with 561 new diagnoses, compared to the pre-pandemic period, with 408 new diagnoses, for an overall increase of 38% (Incidence rate ratio [IRR] 1.38, 95% confidence interval [CI] 1.20–1.58). Of those patients diagnosed with DM during the pandemic period, 97 were tested for COVID, and eight were positive.
T1D
The incidence of T1D was higher during the pandemic, with 402 new diagnoses compared to the 361 new diagnoses before the pandemic, an overall increase of 11% (IRR 1.11, 95% CI 1.01–1.23). The median body mass index (BMI) percentile for patients diagnosed with T1D during the pandemic period was significantly higher compared to those diagnosed during the pre-pandemic period (67.1 vs. 53.4, P = 0.012). The overall rate of DKA at the time of diagnosis was not significantly different between the pre-pandemic and pandemic periods. However, of those who presented in DKA at diagnosis, those during the pandemic were more likely to have severe DKA compared to those who presented in DKA at diagnosis during the pre-pandemic period (odds ratio 1.67, 95% CI 1.07–2.59, P = 0.024).
Age, race, BMI percentile, C-peptide levels, incidence of DKA, degree of severity, and monthly incidence rate of those diagnosed with T1D in 2020, 2021, and 2022, were not noted to have any statistically significant differences when compared within the years of the pandemic.
T2D
The incidence of T2D in our population increased by 238% during the pandemic (n = 159) compared to pre-pandemic (n = 47) (IRR 3.38, 95% CI 2.17–5.28).
During the pandemic and before the pandemic, there was almost equal incidence of T2D in males and females, with 51.1% female in 2017–2019 and 49.7% female in 2020–2022. While not statistically significant (P = 0.397), there was a notable difference in the race of those diagnosed with T2D in 2017–2019 compared to 2020–2022. Before the pandemic, the incidence of T2D in non-Hispanic White patients and non-Hispanic Black patients was equal at 40% for each and 11% for Hispanic patients. During the pandemic, the incidence of T2D in non-Hispanic White patients decreased to 26% and increased in non-Hispanic Black and Hispanic patients to 47% and 15%, respectively.
The patients with a new diagnosis of T2D were noted to be significantly younger during the pandemic compared to the pre-pandemic period, with more than 50% of patients being diagnosed between 11 and 15 years of age during the pandemic (P = 0.015).
When the age, race, BMI percentile, C-peptide levels, incidence of DKA, and degrees of severity were compared for those diagnosed with T2D in 2020, 2021, and 2022, no statistically significant differences were noted. However, the monthly incidence rate of T2D was compared within the pandemic years and notable for the highest rate of incidence during 2021 with a 40% decrease in 2022 (IRR 0.60, 95% CI 0.4 – 0.9).
Pattern of incidence
The average incidence rate by season for type 1 and type 2 diabetes are shown in Figure 1. The lowest rates of T1D incidence occurred during the pre-pandemic period summer, with the highest rate in the fall (IRR 1.68, 95% CI 1.23–2.30), followed by spring (IRR 1.43, 95% CI 1.03–1.97) and winter (IRR 1.40, 95% CI 1.03–1.91). During the pandemic, the highest rates of T1D incidence occurred in the summer (IRR 1.82, 95% CI 1.22–2.72), followed by fall (IRR 1.41, 95% CI 0.92–2.16), winter (IRR 1.39, 95% CI 0.90–2.16), and spring (IRR 1.38, 95% CI 0.93–2.07) [Figure 2]. Following the pandemic onset, the incidence of T2D increased with the highest rates in the summer (IRR 8.45, 95% CI 3.41–20.96), followed by winter (IRR 7.49, 95% CI 2.88–19.47), spring (IRR 6.61, 95% CI 2.7–16.17), and fall (IRR 6.22, 95% CI 2.40–16.13).
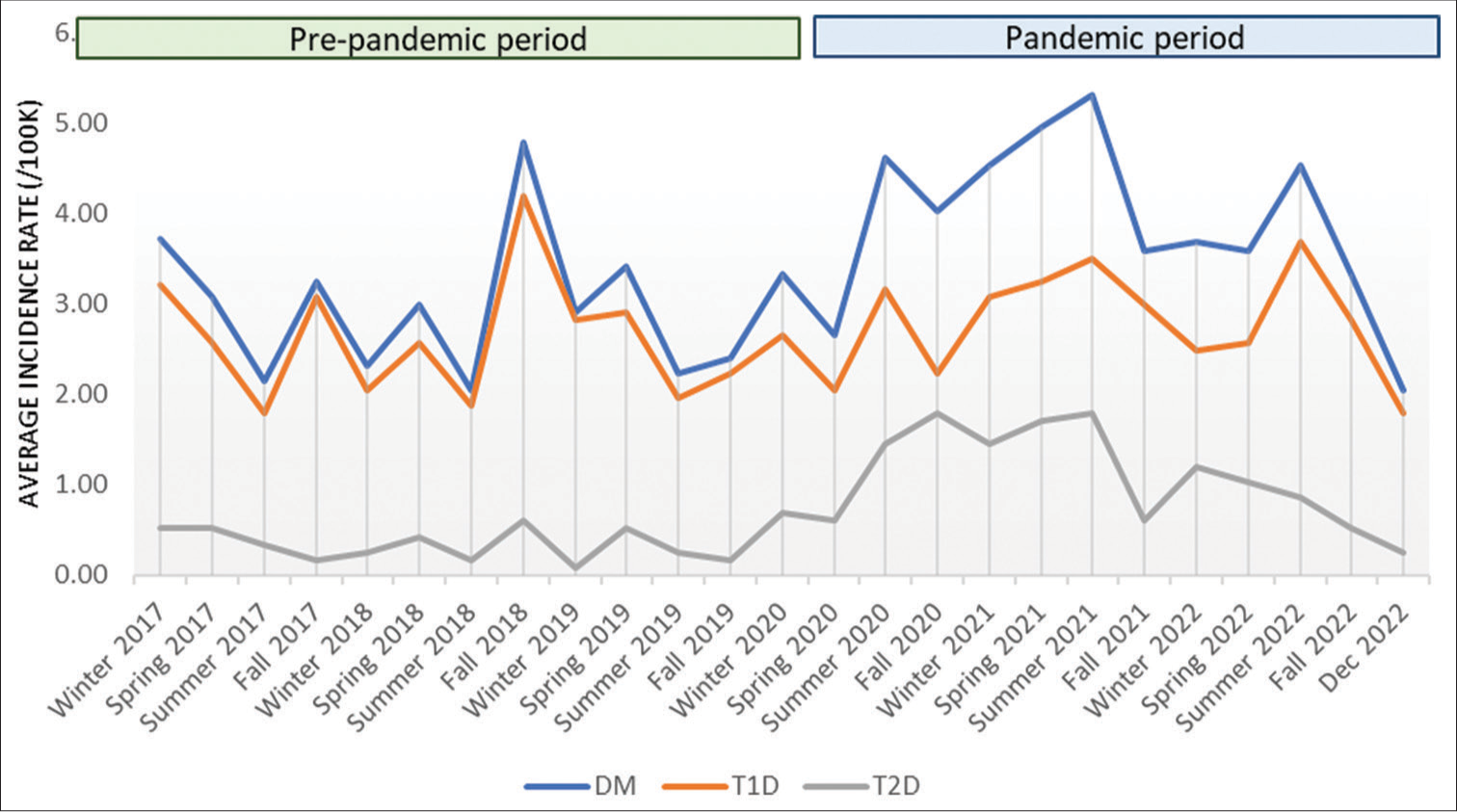
- The average incidence rate by season for all DM, T1D, and T2D from January 2017– December 2022. DM: Diabetes mellitus, T1D: Type 1 diabetes, T2D: Type 2 diabetes.
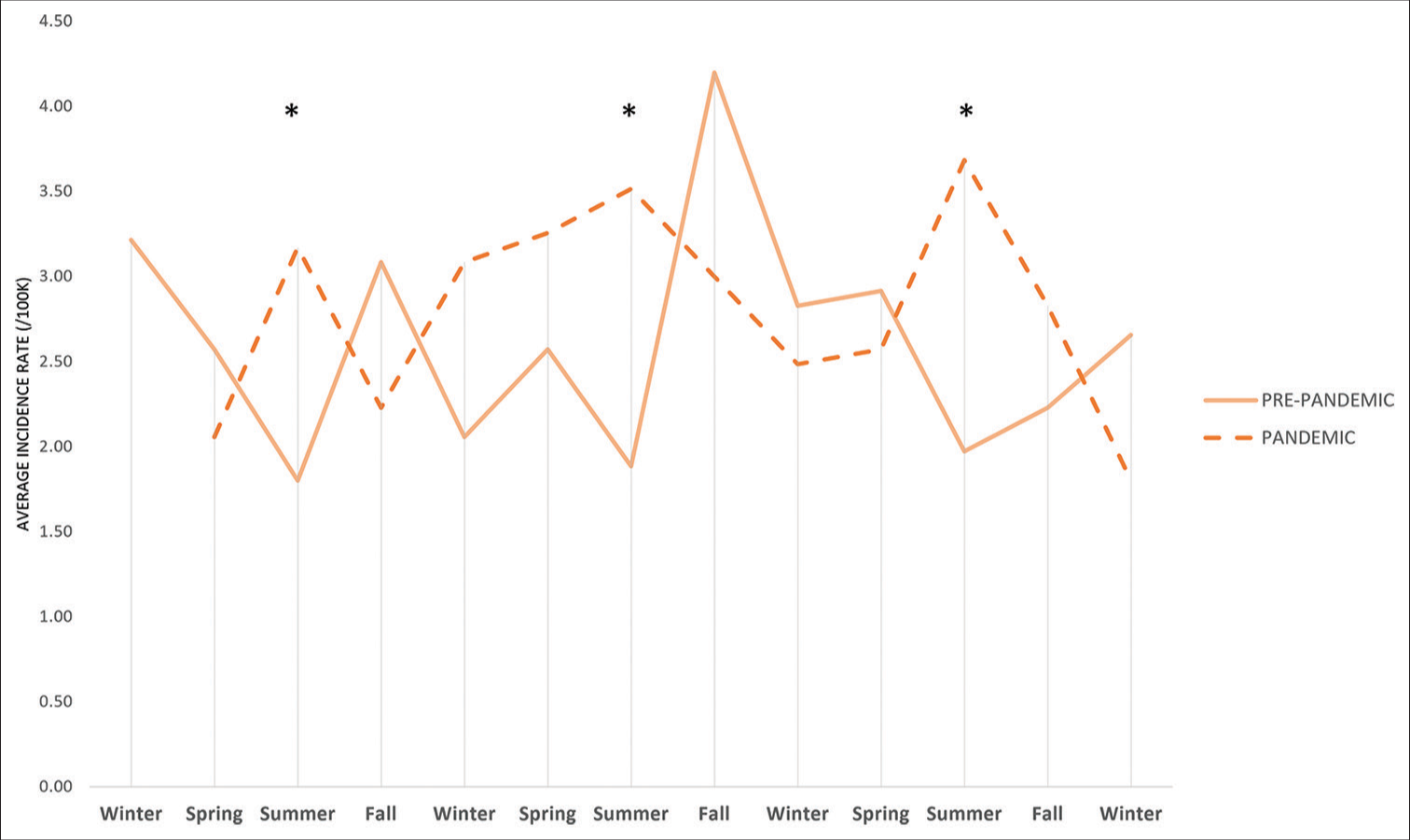
- Comparison of the average seasonal incidence rates of type 1 diabetes between January 2017–February 2020 and March 2020–December 2022. *Incidence rate ratio 1.82 (95% confidence interval 1.22–2.72).
The average incidence trend, adjusted for seasonal patterns and random error, showed an increase during the pandemic to a peak rate for all new diagnoses of DM in March 2021 (4.72/100K), followed by an overall decline until summer 2022 and then stabilized at 3.72/100K [Figure 3]. The graphs for T1D and T2D showed a very similar trendline, with the rate of new diagnosis highest in spring 2021 and then overall decline until spring 2022, after which the trend of new diagnosis stabilized at (2.84/100K, 0.88/100K, respectively).
DISCUSSION
This study identified an increase in the incidence of new-onset T1D and T2D during the pandemic, with a shift in seasonal patterns that persisted through the end of 2022. In concert with what has been reported,[1,5-20,31] the rate of new-onset DM increased in our population during 2020–2021. The monthly incidence trend for T1D and T2D peaked in spring 2021, gradually declined until summer 2022, and then remained stable through December 2022 [Figure 3]. For T2D, the trend stabilized at an incidence rate lower than in spring 2021 but higher than in the pre-pandemic period. The trend rate of new-onset T1D at the end of 2022 is higher than the majority of the pre-pandemic period, with the exception of winter 2018–2019.
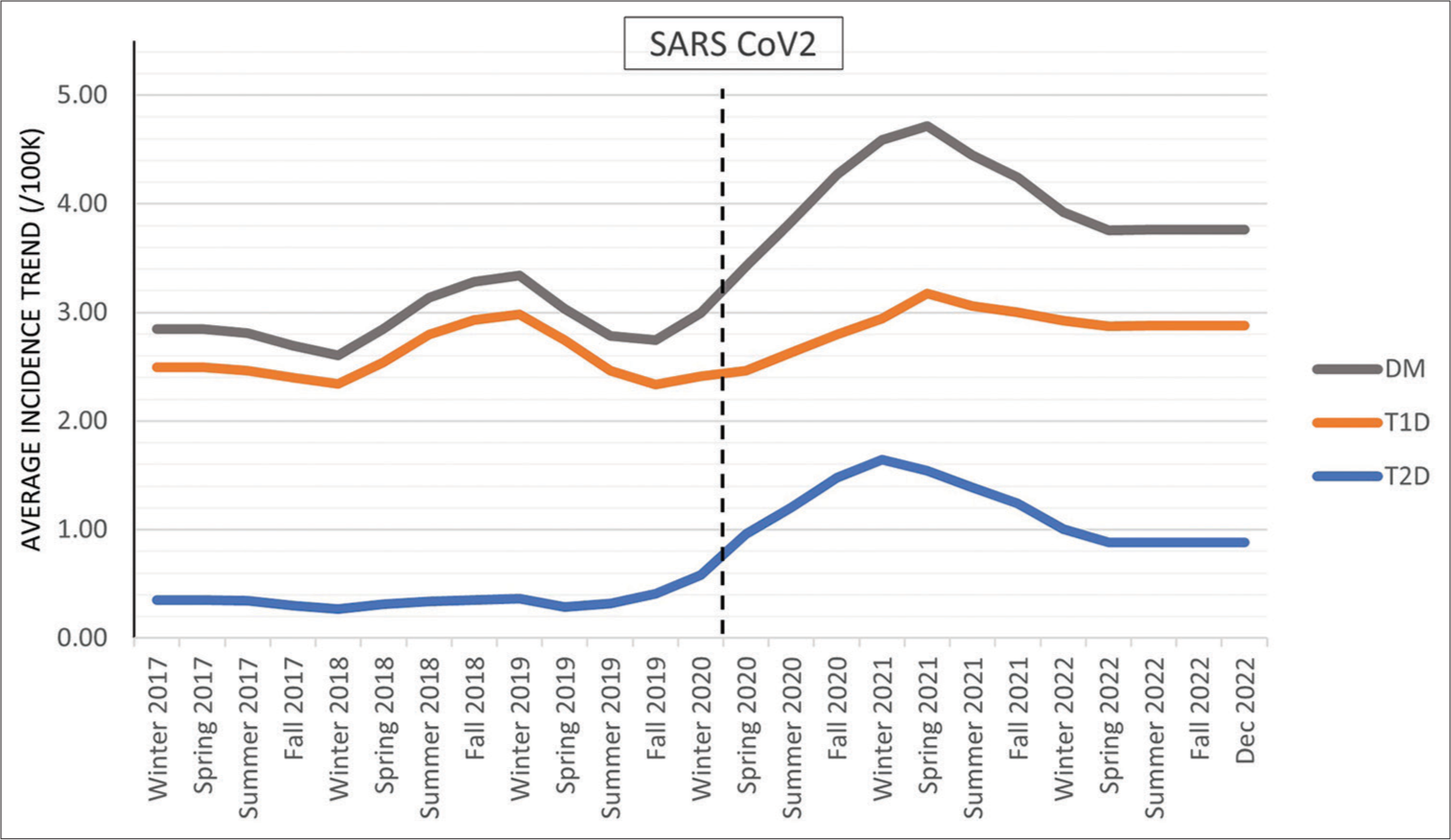
- Graphical representation of the trend based on monthly incidence. The average incidence trend by season for all patients diagnosed with DM, those diagnosed with T1D, and those diagnosed with T2D. DM: Diabetes mellitus, T1D: Type 1 diabetes, T2D: Type 2 diabetes.
To the best of our knowledge, this is the first report of the stabilized incidence for T1D and T2D in 2022. The decrease and stabilization of the incidence of DM in pediatric patients during the second half of 2021 and throughout 2022 was likely multifactorial. The return to in-person school and structured environments with increased opportunities for physical activity may have had positive effects on weight status and metabolic changes that occurred during the sedentary period. In addition, the decrease in rates of COVID infection and social isolation likely led to some improvement in psychological stress. It remains to be seen how this trend will evolve as components of lifestyle and social environments continue to change.
In an effort to understand the increase in incidence of DM since the SARS-CoV2 virus reached pandemic status, prior work has evaluated for COVID infection in patients with new-onset DM. These studies showed very low rates of coinfection with COVID-19 at the time of diagnosis or before diagnosis.[13,16-18,36] In our group, less than 20% of patients diagnosed during the pandemic had COVID test results, and a very small proportion of those tested were positive for COVID-19 infection at the time of DM diagnosis.
Theories not related to the direct effects of SARS-CoV-2 infection have been proposed that include the immunologic and downstream metabolic effects of stress, both psychologic and physiologic, related to the drastic environmental changes put in place to prevent the spread of COVID.[2,5,13,37,38] The lockdown measures led to a significant increase in social isolation, a decrease in physical activity, an increase in sedentary lifestyles, the start of virtual school, and the loss of structured environments. Subsequently, the rates of obesity rose at a more rapid rate than in previous years.[39] Woolford et al. found the greatest increase in obesity during this period (2020–2021) occurred in the younger groups, 5–11 years and 12–15 years, in contrast to the pre-pandemic years when the older adolescents had the highest rates of obesity.[39] In addition to this increase in obesity, there is concern that the increase in stress and sedentary lifestyle, along with the decrease in physical activity, further increased insulin resistance in those already at risk. Consistent with the increase in obesity in the younger groups, the age of T2D diagnosis during the pandemic decreased in our population, with the highest rate of new onsets in the 12–15-year-old group. In addition, our study showed that the patients diagnosed with T1D during the pandemic had a higher BMI percentile compared to those diagnosed before the pandemic. Combined, this supports the idea that stress and environmental changes have an impact on immunologic and metabolic function, making them likely contributors to the development of DM.
Due to the retrospective design, no ZnT8 antibody levels were available. In addition, evaluation of prior COVID-19 infection was limited due to the paucity of data available. As this study includes those diagnosed at a single center, and it will be important to evaluate this in diverse populations. While self-reported race was used, we acknowledge this is a social construct and that differences identified are most likely resultant of social determinants of health. This highlights the importance of further study to better characterize the effect of social determinants of health on the incidence of diabetes in youth.
CONCLUSION
This study supported the reported global increase in the incidence of DM in the pediatric population during the pandemic period and identified a persistent shift in the historical seasonal pattern of incidence. We noted a more pronounced change in the rate of T2D diagnosis that occurred at a younger age and an increased BMI percentile of new-onset T1D, parallel to the increased rate of obesity in younger age groups during the pandemic. Interestingly, we demonstrated a peak incidence of both T1D and T2D in spring 2021, followed by a gradual decline in the rate of new diagnoses until it stabilized in summer 2022 for the remainder of the year. Further studies are needed to evaluate the underlying mechanisms of the persistent increase in incidence.
Ethical approval
The research/study was approved by the Institutional Review Board at the University of Louisville, approval number IRB: 22.0768, dated 21st November 2022.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Type 1 diabetes incidence increased during the COVID-19 pandemic years 2020-2021 in Czechia: Results from a large population-based pediatric register. Pediatr Diabetes. 2022;23:956-60.
- [CrossRef] [PubMed] [Google Scholar]
- Is COVID-19 to Blame? Trends of incidence and sex ratio in youth-onset type 2 diabetes in Germany. Diabetes Care. 2023;46:1379-87.
- [CrossRef] [PubMed] [Google Scholar]
- Trends in incidence of youth-onset type 1 and type 2 diabetes in the USA, 2002-18: Results from the population-based SEARCH for diabetes in Youth study. Lancet Diabetes Endocrinol. 2023;11:242-50.
- [CrossRef] [PubMed] [Google Scholar]
- Seasonality at the clinical onset of type 1 diabetes-lessons from the SWEET database. Pediatr Diabetes. 2016;17(Suppl 23):32-7.
- [CrossRef] [PubMed] [Google Scholar]
- The COVID-19 Pandemic affects seasonality, with increasing cases of new-onset type 1 diabetes in children, from the worldwide SWEET registry. Diabetes Care. 2022;45:2594-601.
- [CrossRef] [PubMed] [Google Scholar]
- Presentations, complications, and challenges encountered during management of type 1 diabetes in Egyptian children during COVID-19 pandemic: A single-center experience. Front Endocrinol (Lausanne). 2022;13:814991.
- [CrossRef] [PubMed] [Google Scholar]
- Sharp rise in new-onset pediatric diabetes during the COVID-19 pandemic. WMJ. 2022;121:177-80.
- [Google Scholar]
- Incidence and presentation of new-onset type 1 diabetes in children and adolescents from Germany during the COVID-19 pandemic 2020 and 2021: Current data from the DPV registry. Diabetes Res Clin Pract. 2023;197:110559.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of diabetes in children and adolescents during the COVID-19 pandemic: A systematic review and meta-analysis. JAMA Netw Open. 2023;6:e2321281.
- [CrossRef] [PubMed] [Google Scholar]
- Changes in the presentation of newly diagnosed type 1 diabetes in children during the COVID-19 pandemic in a tertiary center in Southern Turkey. J Pediatr Endocrinol Metab. 2021;34:1303-9.
- [CrossRef] [PubMed] [Google Scholar]
- Stress hyperglycemia, Diabetes mellitus, and COVID-19 infection: The impact on newly diagnosed type 1 diabetes. Front Clin Diabetes Healthc. 2022;3:818945.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of new-onset type 1 diabetes among US children during the COVID-19 global pandemic. JAMA Pediatr. 2022;176:414-5.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of type 1 diabetes in children and adolescents during the COVID-19 pandemic in Germany: Results from the DPV registry. Diabetes Care. 2022;45:1762-71.
- [CrossRef] [PubMed] [Google Scholar]
- A long-term comparison of presenting characteristics of children with newly diagnosed type 1 diabetes before and during the COVID-19 pandemic. J Clin Res Pediatr Endocrinol. 2022;14:267-74.
- [CrossRef] [PubMed] [Google Scholar]
- SARS-CoV-2 and type 1 diabetes in children in Finland: An observational study. Lancet Diabetes Endocrinol. 2023;11:251-60.
- [CrossRef] [PubMed] [Google Scholar]
- Increase in the diagnosis and severity of presentation of pediatric type 1 and type 2 diabetes during the COVID-19 pandemic. Horm Res Paediatr. 2021;94:275-84.
- [CrossRef] [PubMed] [Google Scholar]
- Disrupted pediatric diabetes trends in the second year of the COVID-19 pandemic. J Endocr Soc. 2023;7:bvad092.
- [CrossRef] [PubMed] [Google Scholar]
- Role of the SARS-CoV-2 virus in the appearance of new onset type 1 diabetes mellitus in children in Gran Canaria, Spain. J Pediatr Endocrinol Metab. 2022;35:393-7.
- [CrossRef] [PubMed] [Google Scholar]
- Pediatric type 2 diabetes presentation during the COVID-19 pandemic. Clin Pediatr (Phila). 2022;61:133-6.
- [CrossRef] [PubMed] [Google Scholar]
- Changes in type 2 diabetes trends in children and adolescents during the COVID-19 pandemic. J Clin Endocrinol Metab. 2022;107:e2777-82.
- [CrossRef] [PubMed] [Google Scholar]
- New-onset type 1 diabetes in children during COVID-19: Multicenter regional findings in the U.K. Diabetes Care. 2020;43:e170-1.
- [CrossRef] [PubMed] [Google Scholar]
- SARS-CoV-2 positive serology and islet autoantibodies in newly diagnosed pediatric cases of type 1 diabetes mellitus: A single-center cohort study. Int J Mol Sci. 2023;24:8885.
- [CrossRef] [PubMed] [Google Scholar]
- Increase in the number of pediatric new-onset diabetes and diabetic ketoacidosis cases during the COVID-19 pandemic. Endocr Pract. 2022;28:479-85.
- [CrossRef] [PubMed] [Google Scholar]
- Spike in diabetic ketoacidosis rates in pediatric type 2 diabetes during the COVID-19 pandemic. Diabetes Care. 2021;44:1451-3.
- [CrossRef] [PubMed] [Google Scholar]
- Increased frequency of diabetic ketoacidosis: The link with COVID-19 pandemic. Front Clin Diabetes Healthc. 2022;3:846827.
- [CrossRef] [PubMed] [Google Scholar]
- Ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the COVID-19 pandemic in Germany. JAMA. 2020;324:801-4.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of COVID-19 and risk of diabetic ketoacidosis in new-onset type 1 diabetes. Pediatrics. 2021;148:e2021050856.
- [CrossRef] [PubMed] [Google Scholar]
- Increased paediatric presentations of severe diabetic ketoacidosis in an Australian tertiary centre during the COVID-19 pandemic. Diabet Med. 2021;38:e14417.
- [CrossRef] [PubMed] [Google Scholar]
- Peculiar characteristics of new-onset Type 1 diabetes during the COVID-19 pandemic. Ital J Pediatr. 2022;48:26.
- [CrossRef] [PubMed] [Google Scholar]
- Delays in presentation of new onset diabetes at the start of the COVID-19 pandemic. R I Med J (2013). 2022;105:46-50.
- [Google Scholar]
- Incidence of new-onset type 1 diabetes during Covid-19 pandemic: A French nationwide population-based study. Diabetes Metab. 2023;49:101425.
- [CrossRef] [PubMed] [Google Scholar]
- The SARS-CoV-2 pandemic is associated with increased severity of presentation of childhood onset type 1 diabetes mellitus: A multi-centre study of the first COVID-19 wave. Diabet Med. 2021;38:e14640.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence, severity, and presentation of type 2 diabetes in youth during the first and second year of the COVID-19 pandemic. Diabetes Care. 2023;46:953-8.
- [CrossRef] [PubMed] [Google Scholar]
- 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S17-38.
- [CrossRef] [PubMed] [Google Scholar]
- ISPAD clinical practice consensus guidelines 2022: Type 2 diabetes in children and adolescents. Pediatr Diabetes. 2022;23:872-902.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-SARSCoV-2 antibodies in new-onset type 1 diabetes in children during pandemic in Belgium. J Pediatr Endocrinol Metab. 2021;34:1319-22.
- [CrossRef] [PubMed] [Google Scholar]
- Examining the associations between COVID-19 infection and pediatric type 1 diabetes. Expert Rev Clin Immunol. 2023;19:489-97.
- [CrossRef] [PubMed] [Google Scholar]
- New-onset type 1 diabetes and severe acute respiratory syndrome coronavirus 2 infection. Immunol Cell Biol. 2023;101:191-203.
- [CrossRef] [PubMed] [Google Scholar]
- changes in body mass index among children and adolescents during the COVID-19 pandemic. JAMA. 2021;326:1434-6.
- [CrossRef] [PubMed] [Google Scholar]







