Translate this page into:
Maternal vitamin D status and its implications on the newborn – A narrative review

*Corresponding author: Sangeeta Yadav, Department of Pediatrics, Hamdard Institute of Medical Sciences and Research, Delhi, India. drsangeetayadav18@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Goswami A, Yadav S. Maternal Vitamin D status and its implications on the newborn – A narrative review. J Pediatr Endocrinol Diabetes. 2024;4:119-28. doi: 10.25259/JPED_55_2024
Abstract
The pandemic of vitamin D deficiency affects all ages, including pregnant women and newborns. The functional significance of maternal and neonatal vitamin D deficiency is incompletely understood. Neonatal vitamin D insufficiency has been linked with preterm birth, seizures, neonatal respiratory distress syndrome, sepsis, acute gastroenterocolitis, and a higher risk of hospital admissions. The potential underlying mechanisms include the effect of vitamin D receptor polymorphism, defective immune effector cells, placental inflammation and gut dysbiosis. About 50% of all neonatal hypocalcemic seizures are attributable to vitamin D deficiency. Serum total calcium levels below 8.0 mg/dL should lead to a high index of suspicion for vitamin D deficiency-related seizure. With appropriate supplementation, hypocalcemic seizures recover without any long-term neurodevelopmental sequelae. Several studies also indicate the benefit of vitamin D supplementation during pregnancy on neonatal anthropometric measures, that is, birth weight (BW), birth length, femur length, head circumference, and ponderal index. The BW has an inverted U shape relationship with vitamin D levels, with benefit observed up to 20 ng/mL. Thus, current evidence underscores the possible detrimental effects of maternal vitamin D deficiency on adverse neonatal outcomes. Hence, maternal vitamin D supplementation may be beneficial for optimal health of the newborns.
Keywords
Birth weight
Bone-mineral homeostasis
Hypocalcemic seizures
Maternal status
Perinatal and neonatal periods
Vitamin D
INRODUCTION
Vitamin D is a fat-soluble vitamin which is necessary for bone mineral homeostasis. Vitamin D status is assessed by serum 25-hydroxyvitamin D [25(OH)D] levels due to its longer half-life of 21 days. About 95% of circulating 25(OH)D is endogenously produced in the skin by the action of ultraviolet rays from sunlight. The pandemic of hypovitaminosis D affects all ages, including pregnant women and their newborns with varied prevalence depending on the cutoffs used.[1-5] Two different cutoffs are currently in use.[4,5] The Endocrine Society, USA recommends circulating 25(OH)D levels >30 ng/mL as sufficient and <20 ng/mL as deficient, whereas Institute of Medicine, USA recommends >20 ng/mL as sufficient and <12 ng/mL as deficient, respectively.[4,5]
CASE SCENARIOS
Vitamin D is often overlooked as a potentially treatable parameter in various neonatal conditions and resultant outcomes. The relationship between vitamin D status and small for gestational age (SGA) is postulated to be mediated by placental vascularization. The impact of vitamin D status on fetal growth is often confounded by perinatal risk factors. Furthermore, the pleiotropic role of vitamin D in immune regulation is often linked to the risk of development of neonatal sepsis. Herein, we describe two babies where hypovitaminosis D was observed in the setting of neonatal sepsis and SGA.
Case 1
A female baby was born at term by lower segment cesarean section to a mother with no apparent risk factors for sepsis. Birth weight (BW) was 3 kg and she was shifted to neonatal intensive care unit for respiratory distress. She was diagnosed with early onset neonatal sepsis as suggested by positive sepsis screen of raised total leukocyte counts and positive C-reactive protein (CRP). She was managed with intravenous antibiotics and discharged on 7th day. Maternal and cord blood 25(OH)D, available in retrospect showed a value of 4.51 and 6.91 ng/mL, respectively.
Case 2
A male baby was born at 38 weeks and 2 days of gestational age by vaginal delivery. Mother had no apparent comorbidities affecting fetal growth during pregnancy. Baby had a BW of 2.51 kg which was <10th centile for gestational age. Serum 25(OH)D levels of mother and cord blood were 3.9 and 4.18 ng/mL, respectively, available in retrospect.
These cases highlight the possible link between vitamin D deficiency in neonatal sepsis and anthropometric parameters including SGA. However, hypovitaminosis D in both these cases was missed in the absence of routine assessment of maternal and newborn 25(OH)D levels. Herein, we summarize a brief review of the relevant studies on the functional significance of vitamin D in neonates.
VITAMIN D STATUS IN INDIAN PREGNANT WOMEN AND NEWBORN DYAD
The exposure of sunlight in India ranges from approximately 3–4 h in winters to 7 h in summer.[6] However, rapid urbanization with prolonged indoor work hours and traditional cultural attire where people cover most body surface have possibly led to further diminutive exposure of sunlight resulting in significant prevalence of hypovitaminosis D in native Indians.[6,7] Vitamin D deficiency is a concern, especially in pregnancy as it potentially affects both mother and fetus. Several studies have shown widespread prevalence of vitamin D deficiency during pregnancy in India.[3-6,8-10] Some of the salient studies are summarized in Table 1. Briefly, Goswami et al., assessed vitamin D status in 29 pregnant women in a public hospital of New Delhi. The mean maternal and cord blood 25(OH)D was 8.8 ± 4.3 and 6.7 ± 2.0 ng/mL, respectively.[6] Subsequently, Sachan et al., reported similar findings for 117 mother and newborn dyads from Lucknow.[8] With increased awareness about hypovitaminosis D, calcium and vitamin D supplementation is being commonly prescribed in India. Recently, Gowtham et al., reported hypovitaminosis D in 65% of mothers and 68.6% newborns in 121 cases from Puducherry, India.[9] A 5-fold higher risk of hypovitaminosis D was observed in newborns of vitamin D deficient mothers. Similar findings were reported from the Middle East, African, and Western countries.[11,12] Goswami et al., in 2016, showed that mother with twin pregnancy had higher vitamin D deficiency with 25(OH)D <12 ng/mL in 89% cases.[13]
| Authors (Reference) | Subjects | (n) | Serum 25(OH)D (ng/mL) | Comments | |
|---|---|---|---|---|---|
| Maternal | Newborn | ||||
| Goswami et al., 2000[6] | Pregnant women belonging to poor socioeconomic status and their newborns | 29 | 8.8±4.3 | 6.7±2.0 | High prevalence of vitamin D deficiency in Asian Indian pregnant women with good correlation between maternal and cord blood 25(OH)D. |
| Sachan et al., 2005[8] | Urban and rural pregnant women at term and their newborns | 117 | 14±9.3 | 8.4±5.7 | 84% prevalence of maternal hypovitaminosis D with good correlation between maternal and cord blood 25(OH)D |
| Sahu et al., 2009[3] | Pregnant women in the second trimester | 139 | 15.12±7.92 | Not done | 74% of pregnant women had vitamin D deficiency. |
| Seth et al., 2009[4] | Healthy lactating mothers and exclusively breastfed infants, 2–24-weeks-old | 180 | 10.9±5.8 | 11.6±8.3 | Vitamin D <10 ng/mL were found in 48% of the mothers and 43% of the infants |
| Jani et al., 2014[5] | 68 affluent and 82 non-affluent healthy pregnant women between 32 and 36 weeks of pregnancy | 150 | Mean (95% confidence interval) Affluent: 11.8 (10.8, 12.9) Non-affluent: 9.8 (9.1, 10.6) |
Not done | 25(OH)D <20 ng/mL in 91% |
| Gowtham et al., 2022[9] | Pregnant women with singleton pregnancy | 121 | Hypovitaminosis D in 65% of mothers and 69% of newborns | ||
| Ravinder et al., 2022[10] | Pregnant women in their last trimester | 100 | 18.61±6.8 | Not done | Serum 25(OH)D level <20 ng/mL in 62% cases |
25(OH)D: 25-hydroxyvitamin D
FUNCTIONAL SIGNIFICANCE OF MATERNAL VITAMIN D DEFICIENCY
Global and Indian data on maternal and newborn vitamin D deficiency and its functional significance are limited.[6-9,13,14] The fetus, deprived of sunlight, depends entirely on maternal supply of calcium and vitamin D. Neonatal hypovitaminosis D has been linked with preterm birth, alteration in bone mineral content, neonatal respiratory distress syndrome (RDS), seizures, sepsis, acute gastroenterocolitis and increased risk of hospitalization.[15,16] Various anthropometric parameters including BW, birth length, head circumference, femur length, and fontanelle size are also being assessed for their relationship with vitamin D.[17,18] Maternal vitamin D status may have long-term implications on the health of offsprings.[14] Vitamin D deficiency during pregnancy causes various manifestations such as childhood obesity and increased risk of recurrent wheeze in early childhood. In a longitudinal follow-up study involving 198 children born in UK, children of vitamin D deficient mothers had reduced whole body and lumbar spine bone mineral density at 9 year of age.[14] Recently, El-Heis et al., showed that vitamin D supplementation reduced the risk of infantile atopic eczema.[19] However, the effect of maternal vitamin D supplementation on neonatal and childhood bone health is variable. In a large randomized placebo-control trial on vitamin D supplementation, there was no difference in neonatal whole body bone mineral content assessed by dual-energy X-ray-absorptiometry.[15] Figure 1 provides the brief depiction of role of vitamin D in various neonatal outcomes.
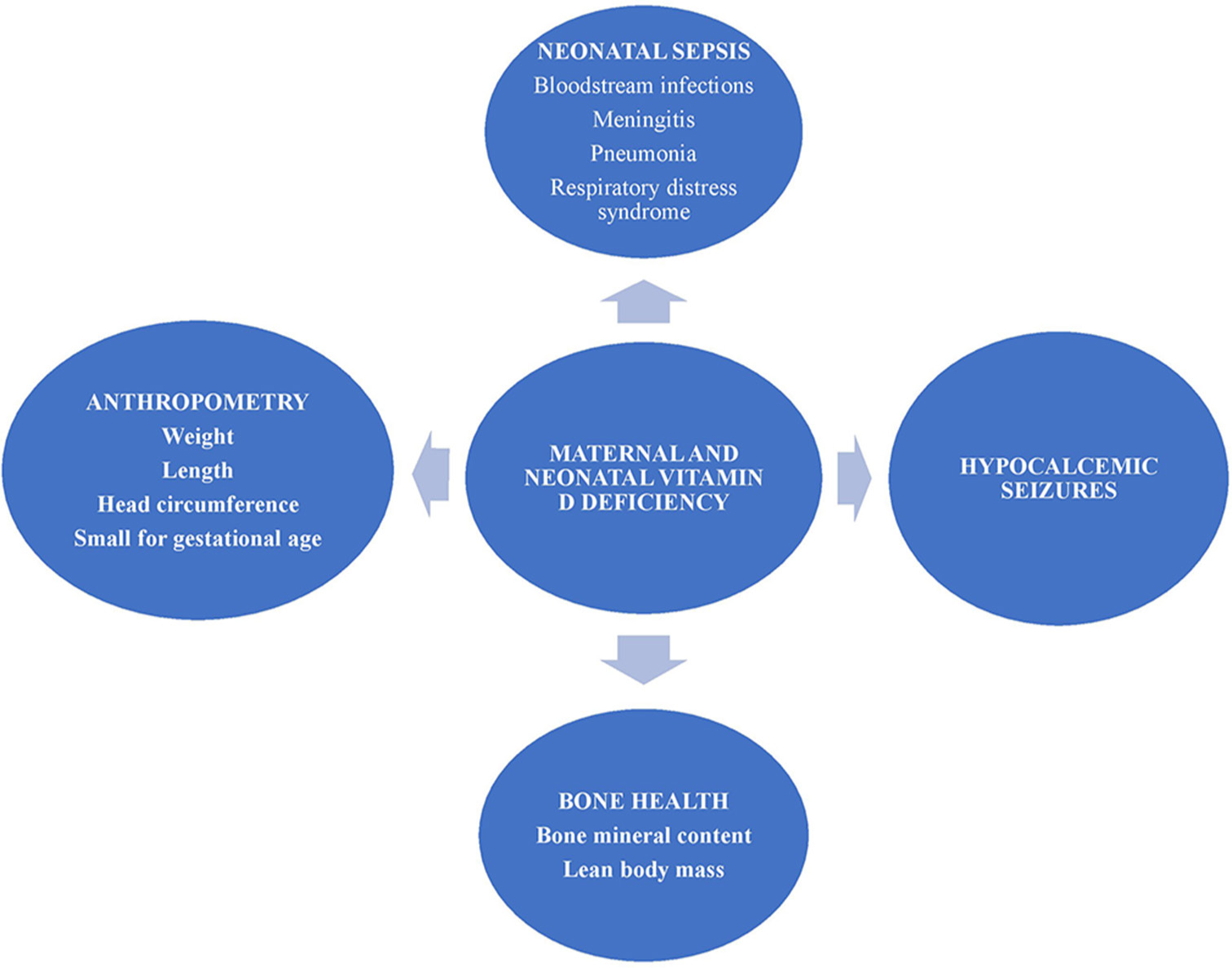
- Salient manifestations of maternal and newborn vitamin D deficiency.
VITAMIN D DEFICIENCY AND NEONATAL SEIZURES
Maternal vitamin D deficiency leads to poor placental transfer of calcium resulting in reduced stores and decreased intestinal calcium absorption in newborns. Decreased serum ionized calcium, in turn, increases the risk of neonatal hypocalcemic seizure.[20-24] The serum total calcium cutoff to define neonatal hypocalcemia ranges from <7.0 to <8.0 mg/dL.[21] Serum ionized calcium cutoff of <4 mg/dL has also been used in disorders affecting albumin levels.[20,21] Immature parathyroid hormone (PTH) response and hyperphosphatemia have been reported in neonatal hypocalcemia.[22] Neonatal hypocalcemia manifests as increased neuromuscular excitability, irritability, tetany, laryngospasm, cardiac arrhythmias, seizures, apnea, cyanosis, and feeding problems.[24]
The prevalence and characteristics of neonatal hypocalcemic seizures have been reported by various investigators. These seizures, occur more commonly in males, and in South Asian or African ethnicity, can be generalized or focal and can occur early at birth, or later, at 4–8 days.[20,23,25] Huang et al., assessed 1029 Taiwanese newborns admitted in intensive care unit and observed neonatal hypocalcemic seizures in 16 cases.[26] Nearly 50% of these seizures were attributed to vitamin D deficiency and remaining to various etiologies including DiGeorge syndrome and hypoparathyroidism. Unlike syndromic cases, those neonates with vitamin D deficiency-related seizures recovered completely with no long-term neurodevelopmental sequelae.[26] SeymenKarabulut et al. assessed 96 Turkish newborns with late-onset neonatal hypocalcemia, occurring at a median age of 5 days with a male preponderance of 60%.[25] The median cord blood 25(OH)D was 6.3 ng/mL with vitamin D deficiency (<12 ng/mL) in 86% and neonatal seizures in 18%. Interestingly, 54.2% of these neonates had low or normal intact PTH (iPTH).[25] Soliman et al. reported cases of neonatal seizures occurring within 10 days of birth.[27] Mothers of affected neonates had 25(OH)D values <10 ng/mL. The seizures were generalized, eight newborns had craniotabes and serum iPTH was normal in 40% of the cases. Treatment with alphacalcidiol and calcium lead to resolution of symptoms within 2 days.[27] Thomas et al. described 78 cases of neonatal hypocalcemia at a children’s medical center in USA.[28] Median age at admission was 8 days, 72% were male, and median duration of hospital stay was 3 days. Majority had hypomagnesemia and low iPTH. The seizures responded to supplementation with calcium, calcitriol, low phosphorus formula, and magnesium.[28]
Although hypovitaminosis D is common in India, the prevalence of neonatal hypocalcemia and seizures is not clear. Mehrotra et al., evaluated vitamin D deficiency in 60 neonates with seizures and their mothers and compared them with healthy neonate-mother pairs in New Delhi, India.[21] Serum 25(OH)D levels were below 10 ng/mL in 90% of newborns with seizures versus 42% in healthy neonates. Around 85% mothers with affected neonates had vitamin D deficiency when compared to 50% in mothers of healthy neonates.
Thus, up to 50% of all neonatal hypocalcemic seizures could possibly be attributed to vitamin D deficiency with a greater risk in males than females. Serum total calcium level <8.0 mg/dL should alert about possibility of a vitamin D deficiency-related seizure. PTH response is often blunted despite severe hypocalcemia due to inadequate maturation. Appropriate treatment leads to complete recovery without long-term neurodevelopmental sequelae.
VITAMIN D DEFICIENCY AND NEONATAL INFECTIONS
Neonatal sepsis manifests within the first 4 weeks of birth. It can occur early within 72 h, or late beyond 72 h of life.[29] Bloodstream infections, meningitis, and pneumonia are common causes of neonatal septicemia. Sepsis results in about 20% of neonatal mortality.[29] Neonatal respiratory diseases also have long-term sequalae, predisposing to respiratory dysfunction, asthma, chronic obstructive disease, and increased mortality at adult age. The burden of neonatal sepsis is high in low-income Asian and African countries.
Several studies have demonstrated possible association between vitamin D and RDS, wheezing, and infections of respiratory tract in infancy.[30] Novel mechanisms are being proposed to explain increased susceptibility to neonatal infections in hypovitaminosis D.[16,31-34]
Recently, Treiber et al., assessed 402 Slovenian mother and newborn dyads.[16] Cord blood 25(OH)D was <10 ng/mL in 18% of newborns. Risk of neonatal RDS, hospitalization, and acute gastroenterocolitis was high in vitamin D deficient state, with odd ratio ranging from 3.9 to 5.9.[16] Similar findings have been reported across India [Table 2]. Dhandai et al., observed significantly low serum 25(OH)D in 60 neonates with late-onset sepsis from a tertiary care center in New Delhi.[35] Singh and Chaudhari examined 70 cases with and without sepsis each, from Surat.[36] Cord blood 25(OH)D was low in 80% of newborns with sepsis unlike in 52% of cases without sepsis, with increased mortality in those with 25(OH)D level <11 ng/mL. Similar observations have also been reported by Behera et al., in culture-positive neonatal sepsis from Odisha[37] and by Agrawal et al., in 225 term neonates from Bhopal.[38] Cases of neonatal sepsis associated with vitamin D deficiency have associated high CRP and longer hospital stay, especially in those with raised serum iPTH.[39,40]
| Authors (Reference) | Subjects | (n) | Comments |
|---|---|---|---|
| Dhandai et al., 2018[35] | Neonates with late onset sepsis (New Delhi, India) | 60 | Infants with neonatal sepsis have significantly low vitamin D. |
| Singh and Chaudhari, 2020[36] | Newborns with and without sepsis (Surat, Gujarat, India) | 70 | Cord blood 25(OH)D lower in 80% of newborns with sepsis and 52% in cases without sepsis. Mortality high when serum 25(OH)D <20 ng/mL. |
| Behra et al., 2020[37] | Newborns with culture positive sepsis. Control newborns without sepsis (Bhubaneswar, Odisha, India) | 40 | 25(OH)D in sepsis group (12.7±2.8 ng/mL). Controls (25.5±7.0 ng/mL). Odds ratio 273 (95% CI 30.39–2451.6) for culture positive sepsis. |
| Agrawal et al., 2019[38] | Term neonates with sepsis and controls without sepsis (Bhopal, Madhya Pradesh, India) | 225 Sepsis=175 Controls=50 |
25(OH)D in cases (12.3±6.1 ng/mL) Controls (14.9±7.2 ng/mL). 86.3% of neonates with sepsis and 74.0% of controls had vitamin D deficiency. |
| Workneh Bitew et al., 2020[29] | Meta-analysis of 40 observational studies including 4 studies from India | 748 with sepsis | 80% of newborn with sepsis were vitamin D deficient and 44% without sepsis. Mean cord 25(OH)D was lower by 8.78 ng/mL in neonatal sepsis |
CI: Confidence interval, 25(OH)D: 25-hydroxyvitamin D
Workneh Bitew et al., reported meta-analysis of 14 observational studies (four from India), which included 748 neonates with sepsis and 573 neonates without sepsis and their respective mothers. About 80% of neonates with sepsis had vitamin D deficiency compared to 44% without sepsis. The mean cord 25(OH)D levels were lower by 8.78 ng/mL in neonatal sepsis. The forest plot showed association of neonatal sepsis with hypovitaminosis D in 13 of the 14 studies.[29]
Information on the maternal vitamin D supplementation and newborn infections is scarce. Loddo et al., in 2023, assessed the effect of antenatal administration of a single dose of 100,000 IU of cholecalciferol.[30] The supplemented cohort of 54,596 subjects with supplementation had 3.0% lower odds of term infants with perinatal asphyxia, respiratory distress, and meconium aspiration syndrome.
While these studies highlight the role of vitamin D deficiency in neonatal sepsis, underlying pathogenetic mechanisms are not completely understood. Tayel et al., observed a high prevalence of FokI TT polymorphism of vitamin D receptor in vitamin D deficient infants with neonatal sepsis, indicating a possible defect in vitamin D action.[31] The effect of vitamin D deficiency on the immune system is being elucidated in both experimental and clinical studies.[32-34] In a mouse model of lung injury, prenatal vitamin D supplementation reduced monocytes/macrophage migration and transforming growth factor–beta-mediated inflammatory pathway activation at the injured site.[32] Wang et al., observed that winter-born neonates had higher risk of vitamin D deficiency, pneumonia, sepsis, cytomegalovirus infection, and low circulating serum CD3+, CD4+, and IgA.[33] Youssef et al. observed similar alterations in effector T-cells in 52 Egyptian neonates with hypovitaminosis D.[34] Zhang et al. observed an association between maternal vitamin D deficiency, placental inflammation, and neonatal sepsis in Chinese women.[41] Intrauterine infection (15.6 vs. 5.7%) and neonatal sepsis (2.4% vs. 0.5%) were higher in mothers with placental inflammation than in those without inflammation. Marsubrin et al., in 2024, assessed the association of vitamin D deficiency with intestinal dysbiosis in 43 preterm infants below 32 weeks.[42] The ratio of fecal commensal Lactobacillaceae to pathogenic Enterobacteriaceae was lower in those with vitamin D deficiency.
Thus, neonates with vitamin D deficiency, especially those with high iPTH, are possibly at higher risk of neonatal sepsis, RDS, wheezing, and prolonged hospital stay. Potential postulated mechanisms include effect of vitamin D receptor polymorphism, defect in immune effector cells, placental inflammation, and gut dysbiosis. Randomized-control trials would help to substantiate the role of prenatal vitamin D supplementation in the prevention of neonatal infections. The ongoing double-blind “D-Kids” trial involving weekly supplementation with 14,000 IU of cholecalciferol in 300 Australian pregnant women will probably help understand the effect of antenatal vitamin D supplementation in neonatal infections.[43]
VITAMIN D AND ANTHROPOMETRY
Anthropometric parameters such as BW, length, head circumference, and anterior fontanelle size reflect the general health and nutritional status of the newborn. The concept of fetal origin of adult onset illnesses suggests that BW is significantly linked to several adult-onset diseases including diabetes mellitus, hypertension, insulin resistance, polycystic ovarian disease, and cardiovascular diseases.[44,45] However, evidence on possible association between maternal and cord blood vitamin D status with anthropometry is conflicting [Table 3].
| Authors (Reference) | Subjects | (n) | Comments |
|---|---|---|---|
| Positive association between maternal 25(OH) D and neonatal anthropometry | |||
| Chen et al., 2024[17] | Chinese women between 16 and 20 weeks of gestation | 510 | Serum 25(OH)D ≤14.7 ng/mL a risk factor for deliveries <38 weeks and BW<3.4 Kg |
| Luo et al., 2022[46] | Chinese women-newborn dyad | 103 | BW lower than 65 g in mothers with 25(OH)D of 15 ng/mL |
| Lee et al., 2022[47] | Mother-newborn dyad, Malaysia | 217 | Maternal 25(OH)D <12 ng/mL led to lower BW, HC and crown heel length |
| Meng et al., 2020[48] | Chinese women in 2nd trimester and their newborns | 3407 | Vitamin D deficient mothers with high PTH had 3 fold risk of SGA newborn with BW lower by 125 g |
| Francis et al., 2018[49] | Mother-newborn dyad, USA | 321 | Obese women with 25(OH)D <20 ng/mL had lower BW and shorter length than non-obese women with similarly low vitamin D |
| Casey et al., 2018[50] | Irish women between 24 and 32 weeks gestation | 1585 | Increased maternal 25(OH)D by two-fold associated with higher BW and length |
| Sarma et al., 2018[51] | Mother-newborn dyad from north-east India | 250 | Vitamin D deficient mother had significantly lower length of their newborn |
| Boghossian et al., 2019[52] | African-American, Caucasian women and their newborns | 343 | Vitamin D deficient male newborns had lower BW and lean body mass by 308 and 217 g, respectively |
| Inverted U shaped relationship between 25(OH) D and neonatal anthropometry | |||
| Keller et al., 2018[53] | Neonates, Germany | 2686 | Inverted U-shaped relation between 225(OH)D with BW and ponderal index. Positive relationship till serum 25(OH)D 20 ng/mL and decline beyond this level |
| Zhu et al., 2015[54] | Neonates, China | 1491 | Every 4 ng/mL increase in cord blood 25(OH)D, increased BW by 61 g, up to a 16 ng/mL. Further increase in 25(OH)D led to a decrease in BW by 68.5 g |
| Lack of relationship between maternal 25(OH) D and neonatal anthropometry | |||
| Yang et al., 2023[55] | Chinese women and their newborns | 199 | No association between cord blood 25(OH)D and BW. |
| Bhowmik et al., 2019[56] | Pregnant women from Bangladesh | 498 | 46% women had 25(OH)D <12 ng/mL. No correlation between cord blood level and BW |
| Yuniati et al. 2020[57] | Indonesian mother-newborn pair | 203 | No correlation between maternal vitamin D status and BW adjusted for maternal age, body weight, and parity |
| Van der Pligt 2023[58] | Vitamin D sufficient Australian mother and their newborns | 221 | No correlation between maternal 25(OH)D and BW and size of newborn. |
BW: Birth weight, HC: Head circumference, SGA: Small for gestational age, g: Grams, PTH: Parathyroid hormone, 25(OH)D: 25-hydroxyvitamin D
Positive association between maternal 25(OH)D and newborn anthropometry
Chen et al., in 2024, assessed vitamin D status of Chinese mothers during pregnancy. Serum 25(OH)D ≤14.7 ng/mL was an independent risk factor for deliveries at ≤38 weeks and BW <3.4 kg.[17] Similarly, Luo et al., reported lower neonatal BW by 65 g in Chinese mothers with a serum 25(OH)D of 15.1 ng/mL.[46] Lee et al., assessed maternal and neonatal vitamin D status and gene polymorphism of vitamin D receptor in Malaysian women.[47] Maternal 25(OH)D <12 ng/mL was associated with lower BW, head circumference, and crown−heel length. Fok 1 polymorphism showed an additive effect on reduced head circumference. This interaction between vitamin D receptor gene and vitamin D nutrition may affect anthropometric parameters at birth.[47] Meng et al., observed that mothers with high iPTH levels had 3-fold higher risk of SGA newborns, and BW lower by 125 g.[48]
Maternal body mass index (BMI) before pregnancy can confound the effect of hypovitaminosis D on fetal anthropometry. Francis et al., assessed 321 maternal-newborn pairs in the USA and observed that obese women with 25(OH)D <20 ng/mL had lower and shorter lengths of their newborns as compared to newborns of women with normal BMI.[49] Casey et al. observed that higher maternal serum 25(OH)D by two-fold in second trimester in Irish women was associated with higher BW and length standard-deviation score, by 0.05 and 0.07, respectively.[50]
Sarma et al., assessed 250 primigravida and their newborns who had mean 25(OH)D of 17.5 ± 2.2 ng/mL and 14.5 ± 1.8 ng/mL, respectively.[51] Fetal length at birth including femur length was significantly shorter in newborns of vitamin D deficient mothers. Boghossian et al. reported that vitamin D deficient male newborns of African-American and Caucasian women had lower BW and lean body mass by 308 and 217 g, respectively.[52] The newborns of women with serum total calcium in the lowest tertile had a lower ponderal index and bone mineral density. These studies indicate a probable non-linear relationship between vitamin D levels and BW of the newborns.
U-shaped association between 25(OH)D and newborn anthropometry
Keller et al. reported an “inverted U-shaped” association of neonatal 25(OH)D with BW and ponderal index. A positive relation of the parameters with 25(OH)D is observed with up to <20 ng/mL, with a decline beyond this level.[53] Similarly, Zhu et al., reported that every 4 ng/mL increase in cord blood 25(OH)D levels led to increase in BW by 61 g up to a level of 16 ng/mL.[54] Further increase in 25(OH)D led to a decrease in BW by 68.5 g.
Lack of association between maternal 25(OH)D and newborn anthropometry
Several studies indicate lack of beneficial effect of maternal vitamin D supplementation on anthropometry of newborns.[55-59] Yang et al., in 2023, reported lack of relationship between cord blood 25(OH)D and small (SGA), appropriate, and large for gestational age newborns among Chinese women.[55] Bhowmik et al., in 2019, observed serum 25(OH)D <12 ng/mL in 46% of 498 pregnant women in Bangladesh, with no correlation observed between BW and cord blood 25(OH)D.[56] Similarly, Yuniati et al. observed lack of relationship between maternal vitamin D levels and BW of newborn adjusted for maternal age, body weight, and parity in Indonesian mother-newborn pairs.[57] Van der Pligt et al., also, did not observe significant correlation between serum 25(OH)D and BW and size of newborns in vitamin D-sufficient Australian women, with serum 25(OH) D >33 ng/mL.[58]
Given the importance of 25(OH)D in bone-mineral homeostasis, maternal and neonatal vitamin D status may determine fontanelle size in neonates. Cho et al., 2023 observed that among 18 infants with large anterior fontanelle, 16 had decreased serum vitamin D levels.[18] A randomized-control trial Brooke et al., reported that newborns of vitamin D supplemented mothers had smaller anterior fontanelle size.[60] Similarly, Kalra et al., observed that maternal vitamin D supplementation in doses varying from 60,000 IU to 120,000 IU, was associated with anterior fontanelle size of 2.5 cm as compared to 3.3 cm in the non-supplemented group.[61] It seems that hypovitaminosis D is associated with larger anterior fontanelle size that can be corrected by its supplementation.
In summary, various studies indicate a possible beneficial effect of vitamin D on neonatal BW, length, femur length, head circumference, ponderal index, lean body mass, and bone density. The BW seems to follow an inverted U shape with vitamin D, with maximum benefit observed at approximately 20 ng/mL. Role of vitamin D on fetal anthropometry is not completely understood. vitamin D increases the lean mass and bone mineral density, indicating its direct effect through parameters of bone mineral homeostasis.
There is a lack of prospective studies on the role of vitamin D supplementation in determining neonatal anthropometric parameters. Some studies have indicated a beneficial effect of vitamin D on BW and size.[62,63] Tao et al. assessed the effect of antenatal cord blood supplementation with 600 IU/day of cholecalciferol for at least 2 months.[62] Mean 25(OH)D was higher by 1.4 ng/mL in the supplemented group. However, even with this modest improvement, the risk of SGA newborns decreased by nearly two-fold from 11.8% to 6.9%. Similarly, Kılıcaslan et al., observed improved head and chest circumference and height of newborns of 100 Turkish women who had received vitamin D supplementation.[63] Kalra et al., reported that antenatal vitamin D supplementation with either one oral dose of 60,000 IU or two doses of 120,000 IU led to increased head circumference, length, and weight of newborns.[61] However, intermitted doses of vitamin D are not recommended in neonates.[64,65]
CONCLUSION
Thus, current evidence indicates that Vitamin D deficiency may be associated with adverse neonatal outcome in the perspective of hypocalcemic seizures, sepsis, and anthropometry. The guidelines from the Indian Academy of Pediatrics (IAP) which recommend physiological maintenance dose of 400 IU of 25-hydroxycholecalciferol in all asymptomatic neonates till 1 year of age should be followed. Neonates with hypovitaminosis D should be managed with daily doses of 2000 IU of vitamin D for a period of 3 months along with calcium supplementation. However, further prospective studies with vitamin D supplementation are required to assess its role in neonatal seizures, infection, and anthropometry.
Author’s contributions
Both the authors contributed in the concept, design, intellectual content, literature search, manuscript preparation, editing, and review. Both the authors are guarantors of the work.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
Dr. Sangeeta Yadav is on the Editorial Board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-30.
- [CrossRef] [PubMed] [Google Scholar]
- The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-8.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin D deficiency in rural girls and pregnant women despite abundant sunshine in northern India. Clin Endocrinol (Oxf). 2009;70:680-4.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin D nutritional status of exclusively breast fed infants and their mothers. J Pediatr Endocrinol Metab. 2009;22:241-6.
- [CrossRef] [PubMed] [Google Scholar]
- Widespread 25-hydroxyvitamin D deficiency in affluent and nonaffluent pregnant Indian women. Biomed Res Int. 2014;2014:892162.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and significance of low 25-hydroxyvitamin D concentrations in healthy subjects in Delhi. Am J Clin Nutr. 2000;72:472-5.
- [CrossRef] [PubMed] [Google Scholar]
- Absence of vitamin D deficiency among common outdoor workers in Delhi. Clin Endocrinol (Oxf). 2019;91:356-62.
- [CrossRef] [PubMed] [Google Scholar]
- High prevalence of vitamin D deficiency among pregnant women and their newborns in northern India. Am J Clin Nutr. 2005;81:1060-4.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of maternal hypovitaminosis D on birth and neonatal outcome-a prospective cohort study. J Matern Fetal Neonatal Med. 2022;35:9940-7.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of vitamin D deficiency among South Indian pregnant women. J Family Med Prim Care. 2022;11:2884-9.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal and neonatal vitamin D status at birth in black South Africans. S Afr Med J. 2019;109:807-13.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal and cord blood vitamin D status and childhood infection and allergic disease: A systematic review. Nutr Rev. 2016;74:387-410.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal and neonatal vitamin-D status in twin versus singleton pregnancies. J Obstet Gynaecol Res. 2016;42:1250-7.
- [CrossRef] [PubMed] [Google Scholar]
- Princess Anne Hospital Study Group Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: A longitudinal study. Lancet. 2006;367:36-43.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): A multicentre, double-blind, randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2016;45:393-402.
- [CrossRef] [PubMed] [Google Scholar]
- Association between umbilical cord vitamin D levels and adverse neonatal outcomes. J Int Med Res. 2020;48:300060520955001.
- [CrossRef] [PubMed] [Google Scholar]
- Predictive value of vitamin D levels in pregnant women on gestational length and neonatal weight in China: A population-based retrospective study. Reprod Biol Endocrinol. 2024;22:102.
- [CrossRef] [PubMed] [Google Scholar]
- Anterior fontanel size in Korean nursery newborns and clinical implications of large anterior fontanel: A retrospective cohort, observational study. Medicine (Baltimore). 2023;102:e33882.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal antenatal vitamin D supplementation and offspring risk of atopic eczema in the first 4 years of life: Evidence from a randomized controlled trial. Br J Dermatol. 2022;187:659-66.
- [CrossRef] [PubMed] [Google Scholar]
- Global consensus recommendations on prevention and management of nutritional rickets. Horm Res Paediatr. 2016;85:83-106.
- [CrossRef] [PubMed] [Google Scholar]
- Hypovitaminosis D and hypocalcemic seizures in infancy. Indian Pediatr. 2010;47:581-6.
- [CrossRef] [PubMed] [Google Scholar]
- Hypocalcemic focal seizures in a one-month-old infant of a mother with a low circulating level of vitamin D. Brain Dev. 1991;13:132-4.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of hypocalcemic seizures due to vitamin D deficiency in children in the United Kingdom and Ireland. J Clin Endocrinol Metab. 2015;100:E91-5.
- [CrossRef] [PubMed] [Google Scholar]
- Laryngospasm and neonatal seizure due to hypocalcemia and vitamin D deficiency: An emergency condition in NICU and challenge to the neonatologist. BMJ Case Rep. 2014;2014:bcr2014206795.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin D Deficiency prevalence in late neonatal hypocalcemia: A multicenter study. J Clin Res Pediatr Endocrinol. 2021;13:384-90.
- [CrossRef] [PubMed] [Google Scholar]
- Syndromic and non-syndromic etiologies causing neonatal hypocalcemic seizures. Front Endocrinol (Lausanne). 2022;13:998675.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical, biochemical, and radiological manifestations of vitamin D deficiency in newborns presented with hypocalcemia. Indian J Endocrinol Metab. 2013;17:697-703.
- [CrossRef] [PubMed] [Google Scholar]
- Transient neonatal hypocalcemia: Presentation and outcomes. Pediatrics. 2012;129:e1461-7.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of vitamin D on neonatal sepsis: A systematic review and meta-analysis. Food Sci Nutr. 2020;9:375-88.
- [CrossRef] [PubMed] [Google Scholar]
- Association of Maternal gestational vitamin D supplementation with respiratory health of young children. Nutrients. 2023;15:2380.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin D deficiency and vitamin D receptor variants in mothers and their neonates are risk factors for neonatal sepsis. Steroids. 2018;134:37-42.
- [CrossRef] [PubMed] [Google Scholar]
- Prenatal vitamin D supplementation mitigates inflammation-related alveolar remodeling in neonatal mice. Am J Physiol Lung Cell Mol Physiol. 2023;325:L95-103.
- [CrossRef] [PubMed] [Google Scholar]
- Serum vitamin D insufficiency in hospitalized full-term neonates at a tertiary hospital in Eastern China. Front Pediatr. 2022;10:878992.
- [CrossRef] [PubMed] [Google Scholar]
- In neonates with vitamin D deficiency, low lymphocyte activation markers are risk factors for infection. Paediatr Int Child Health. 2019;39:111-8.
- [CrossRef] [PubMed] [Google Scholar]
- Association of vitamin D deficiency with an increased risk of late-onset neonatal sepsis. Paediatr Int Child Health. 2018;38:193-7.
- [CrossRef] [PubMed] [Google Scholar]
- Association of early-onset sepsis and vitamin D deficiency in term neonates. Indian Pediatr. 2020;57:232-4.
- [CrossRef] [PubMed] [Google Scholar]
- Is Lower Vitamin D level associated with increased risk of neonatal sepsis? A prospective cohort study. Indian J Pediatr. 2020;87:427-32.
- [CrossRef] [PubMed] [Google Scholar]
- Role of Vitamin D deficiency in term neonates with late-onset sepsis: A case-control study. J Trop Pediatr. 2019;65:609-16.
- [CrossRef] [PubMed] [Google Scholar]
- Association of low vitamin D level and full-term early-onset neonatal sepsis; a case-control study. Ital J Pediatr. 2024;50:101.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin D deficiency with high parathyroid hormone levels is related to late onset SEPSIS among preterm infants. BMC Pregnancy Childbirth. 2023;23:23.
- [CrossRef] [PubMed] [Google Scholar]
- Severe vitamin D deficiency in the first trimester is associated with placental inflammation in high-risk singleton pregnancy. Clin Nutr. 2019;38:1921-6.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin D and gut microbiome in preterm infants. BMC Pediatr. 2024;24:588.
- [CrossRef] [PubMed] [Google Scholar]
- Randomised controlled trial of perinatal vitamin D supplementation to prevent early-onset acute respiratory infections among Australian First Nations children: The 'D-Kids' study protocol. BMJ Open Respir Res. 2023;10:e001646.
- [CrossRef] [PubMed] [Google Scholar]
- Barker hypothesis and hypertension. Front Public Health. 2022;9:767545.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of prenatal hypoxia on nervous system development and related diseases. Front Neurosci. 2021;15:755554.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal vitamin D levels correlate with fetal weight and bone metabolism during pregnancy: A materno-neonatal analysis of bone metabolism parameters. J Perinat Med. 2022;51:538-45.
- [CrossRef] [PubMed] [Google Scholar]
- Interplay between maternal and neonatal vitamin D deficiency and vitamin D-related gene polymorphism with neonatal birth anthropometry. Nutrients. 2022;14:564.
- [CrossRef] [PubMed] [Google Scholar]
- The role of parathyroid hormone during pregnancy on the relationship between maternal Vitamin D deficiency and fetal growth restriction: A prospective birth cohort study. Br J Nutr. 2020;124:432-9.
- [CrossRef] [PubMed] [Google Scholar]
- Longitudinal maternal vitamin D status during pregnancy is associated with neonatal anthropometric measures. Nutrients. 2018;10:1631.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal vitamin D and neonatal anthropometrics and markers of neonatal glycaemia: Belfast Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study. Br J Nutr. 2018;120:74-80.
- [CrossRef] [PubMed] [Google Scholar]
- Fetal skeletal size and growth are relevant biometric markers in vitamin D deficient mothers: A North East India prospective cohort study. Indian J Endocrinol Metab. 2018;22:212-6.
- [CrossRef] [PubMed] [Google Scholar]
- Longitudinal measures of maternal vitamin D and neonatal body composition. Eur J Clin Nutr. 2019;73:424-31.
- [CrossRef] [PubMed] [Google Scholar]
- Concentration of 25-hydroxyvitamin D from neonatal dried blood spots and the relation to gestational age, birth weight and Ponderal Index: The D-tect study. Br J Nutr. 2018;119:1416-23.
- [CrossRef] [PubMed] [Google Scholar]
- Cord Blood 25-hydroxyvitamin D and fetal growth in the China-Anhui birth cohort study. Sci Rep. 2015;5:14930.
- [CrossRef] [PubMed] [Google Scholar]
- The association between umbilical cord blood fat-soluble vitamin concentrations and infant birth weight. Front Endocrinol (Lausanne). 2023;14:1048615.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal BMI and nutritional status in early pregnancy and its impact on neonatal outcomes at birth in Bangladesh. BMC Pregnancy Childbirth. 2019;19:413.
- [CrossRef] [PubMed] [Google Scholar]
- First trimester maternal vitamin D, ferritin, hemoglobin level and their associations with neonatal birthweight: Result from cohort study on vitamin D status and its impact during pregnancy and childhood in Indonesia. J Neonatal Perinatal Med. 2020;13:63-9.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal plasma vitamin D levels across pregnancy are not associated with neonatal birthweight: Findings from an Australian cohort study of low-risk pregnant women. BMC Pregnancy Childbirth. 2023;23:67.
- [CrossRef] [PubMed] [Google Scholar]
- Association of maternal circulating 25(OH)D and calcium with birth weight: A mendelian randomisation analysis. PLoS Med. 2019;16:e1002828.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin D supplements in pregnant Asian women: Effects on calcium status and fetal growth. Br Med J. 1980;280:751-4.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of vitamin D supplementation during pregnancy on neonatal mineral homeostasis and anthropometry of the newborn and infant. Br J Nutr. 2012;108:1052-8.
- [CrossRef] [PubMed] [Google Scholar]
- Current recommended vitamin D prenatal supplementation and fetal growth: Results from the China-Anhui birth cohort study. J Clin Endocrinol Metab. 2018;103:244-52.
- [CrossRef] [PubMed] [Google Scholar]
- The effects of vitamin D supplementation during pregnancy and maternal vitamin D levels on neonatal Vitamin D levels and birth parameters. J Matern Fetal Neonatal Med. 2018;31:1727-34.
- [CrossRef] [PubMed] [Google Scholar]
- Prevention and treatment of vitamin D and calcium deficiency in children and adolescents: Indian Academy of Pediatrics (IAP) guidelines. Indian Pediatr. 2017;54:567-73.
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines on prevention and treatment of vitamin D deficiency and rickets. Indian Pediatr. 2022;59:142-58.
- [CrossRef] [PubMed] [Google Scholar]






