Translate this page into:
Impact of delayed diagnosis on catch-up growth of children and adolescents with primary hypothyroidism due to Hashimoto’s thyroiditis

*Corresponding author: Hemchand Krishna Prasad, Department of Pediatric Endocrinology, Mehta Multispeciality Hospitals India Private Limited, Chennai, Tamil Nadu, India. pediatricendocrinology.mehta@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Madu A, Prasad HK, Thiagarajan A, Narayanasamy K, Krishnamoorthy N. Impact of delayed diagnosis on catch-up growth of children and adolescents with primary hypothyroidism due to Hashimoto’s thyroiditis. J Pediatr Endocrinol Diabetes 2021;1:14-9.
Abstract
Objectives:
There is a paucity of data on impact of therapy of Hashimoto’s thyroiditis (HT) on catch-up growth. The objective of the study was to determine whether delayed diagnosis of HT and overt primary hypothyroidism has an impact on the catch-up of children and adolescents.
Material and Methods:
We conducted a prospective observational study over 3 years, in the thyroid clinic of a referral unit in South India. We assessed chronological age (CA), auxological parameters, clinical presentation, bone age (BA), and predicted adult height (PAH) in subjects with Hashimoto’s thyroiditis and overt primary hypothyroidism. Management and follow-up done as per standard protocols and study parameters reassessed after 1 year of therapy.
Results:
We recruited 38 subjects and divided them into two groups – Group 1 with BA within 2 standard deviations (SD) of CA (n = 20) and Group 2 beyond 2 SD (n = 18). During 1 year follow-up, height Z-scores were −0.1 ± 1.4 (baseline) and −0.1 ± 1.1 (endpoint) and −1.7 ± 1.7 (baseline) and −1.3 ± 1.3 (endpoint) in Groups 1 and 2, respectively. BA: CA ratio changed from 1.0 ± 0.1 to 1.0 ± 0.1 in Group 1 (P > 0.05) versus 0.7 ± 0.2 to 0.9 ± 0.1 in Group 2 (P < 0.05). The number of children who were pre-pubertal: pubertal changed from 15:5 to 11:9 in Group 1 and 14:4 to 7:11 in Group 2. For Group 1, baseline PAH Z score was −0.5 ± 1.7 and endpoint PAH Z score was −0.7 ± 1.6 versus a target height Z score of −1.1 ± 1.1 (P > 0.05); Group 2, the baseline PAH Z score −1.1 ± 1.6 and endpoint PAH Z score −2.2 ± 1.4 versus target height Z-score of −0.4 ± 1.7.
Conclusion:
Delayed diagnosis and treatment of juvenile autoimmune hypothyroidism results in permanent loss of height potential.
Keywords
Hashimoto’s thyroiditis
Predicted adult height
Bone age
INTRODUCTION
Juvenile-acquired hypothyroidism is a simple treatable condition that is often encountered and managed by pediatricians and physicians more than the endocrinologists. Hashimoto’s thyroiditis (HT) is the most common cause for acquired hypothyroidism and goiter in children and adolescents. Furthermore, it is the most prevalent autoimmune thyroid gland disease.[1] Juvenile-acquired hypothyroidism has an insidious onset characterized by gradual deceleration and eventual cessation of growth.[2] Untreated children have delayed puberty and undergo epiphyseal fusion resulting in extraordinarily short stature at maturity.[3] However, if thyroxine treatment is started before the epiphyseal fusion, rapid catch-up growth occurs and these children reach normal adult height.[4,5]
Analysis of long-term growth in children with congenital hypothyroidism suggests that catch-up is limited when treatment is delayed.[6] A few studies from the western world have demonstrated that children who are diagnosed late with HT and supplemented with thyroxine show rapid catch-up growth initially in the form of accelerated skeletal development, whereas the final height achieved at maturity is compromised. The permanent height deficit is directly proportional to the duration of thyroxine deficiency before treatment.[7,8] Gonadotropin analog therapy has been reported to improve the final height in children who present late with HT.[9] A recent Indian study has demonstrated that thyroxine therapy for 6 months results in advancement of skeletal maturity by 11 months.[10] Although there are Indian studies on prevalence of HT in children,[2,11] there are no studies describing the impact of therapy on final height potential. Hence, we conducted this study with an aim to determine whether delayed diagnosis of primary hypothyroidism due to HT has an impact on catch-up growth of children and adolescents.
MATERIAL AND METHODS
After approval from the Institutional Review Board, we conducted a prospective observational study over a period of 3 years, from 2016 to 2019. We recruited subjects referred to thyroid clinic of a tertiary referral hospital in South India. We included subjects aged 5–18 years with primary overt hypothyroidism due to HT, after, informed parental consent. Subjects with late presentation of congenital hypothyroidism, concomitant autoimmune disorders such as type 1 diabetes mellitus and celiac disease, and syndromic conditions such as Down’s syndrome were excluded from the study. Diagnosis of HT was based on the classic criteria: Presence of thyroid peroxidase (TPO) antibody and/or thyroglobulin (TG) antibody, associated with primary hypothyroidism.[11]
Free T4 and thyroid-stimulating hormone (TSH) were measured by electrochemiluminescent (Elecsys FT4) assay using a specific anti T4 antibody labeled with a ruthenium complex (limits of detection: 0.5 pmol/L–100 pmol/L) and monoclonal antibody directed against human TSH (0.005 μIU/mL–100 μIU/mL) using Cobas e411 analyzer, respectively. Anti-TPO antibody and anti-TG antibody were measured by electrochemiluminescent immunoassay using a recombinant antigen with polyclonal anti-TPO antibody (limits of detection: 5–600 IU/mL) and human antigen and monoclonal anti-TG antibody (limits of detection: 10–4000 IU/mL), respectively, using Cobas e411 analyzer; test number 720. Interassay and intra-assay coefficient of variance for all biochemical parameters were <10%. TSH value >10 μIU/mL and free T4 <0.9 ng/dL were considered as abnormal. Subjects were recruited if the TSH was >10 μIU/mL (irrespective of level of free T4).[12]
The baseline auxological parameters (height, weight, and body mass index [BMI]) were recorded and sexual maturity rating was assessed by a single clinician. Subjects were ascertained for clinical features of hypothyroidism and goiter was staged as per the World Health Organization recommendation.[13] Z-scores of anthropometric measures were calculated for all children using the IAP 2015 growth charts and Indian growth velocity charts.[14,15] Height of both the parents was recorded and mid-parental height was calculated. Bone age (BA) estimation was done by a single pediatric radiologist using Greulich-Pyle atlas.[16] Predicted adult height (PAH) was estimated using the Bayley-Pinneau tables.[17]
Thyroxine replacement was initiated at a dose of 4 μg/kg/day, 3 μg/kg/day, and 2 μg/kg/day in children ages <6 years, 6–11 years, and >11 years, respectively.[18] The first follow-up was done after 6 weeks and the necessary dose adjustments were made. They were later escalated or reduced based on clinical and biochemical response.[19,20] Uniform treatment strategy was followed for all. Compliance was ensured by regular telephone calls to the parents and assessment of pill count on follow-up visits. Children were followed up 3 monthly for a period of 1 year in the thyroid clinic. They were assessed for growth, sexual maturity, clinical features of hypothyroidism, and biochemical thyroid function testing. After 1 year, all the children were reviewed and the anthropometric parameters were recorded again and plotted in growth charts. BA was reestimated and PAH was recalculated. To assess the tempo of progress and residual growth potential, we used Pace, defined as Δ BA/ΔCA, that is, (endpoint BA-initial BA)/(endpoint CA-initial CA).[21] The baseline and endline Z-scores for height, weight, BMI, and PAH were assessed. All the study subjects continued to be followed up in the thyroid clinic.
Statistical analysis
Statistical analysis was done using version 22.0 of SSPS software. Assuming the effect size (d) of 0.92 for HT for both groups in heights, with 5% alpha level, power of 80%, the minimum sample required for study was 38.[7] Data were entered into Excel sheet. Variables have been described in terms of percentage, proportions, and mean with standard deviation (SD). The study parameters were compared using Chi-square test for proportions, Mann–Whitney U-test and Wilcoxon sign-rank test for continuous variables, as appropriate. Spearman correlation analysis was performed between continuous variables. P <0.05 was considered as statistically significant.
RESULTS
During the study period, 108 children with HT were referred to the endocrine clinic. We recruited 38 subjects who satisfied the inclusion criteria and completed 1 year of follow up. Based on skeletal maturity at baseline, study subjects were divided into two groups – Group 1 and Group 2. Subjects who had BA within 2 SDs of chronological age (CA) constituted Group 1 and those with BA which was 2 SD beyond the CA constituted Group 2.[22] The symptomatology and biochemical parameters are presented in Table 1. The baseline demographic parameters, clinical parameters, height, weight, BMI, sexual maturity rating, target height, skeletal maturity, and PAH in the two groups are presented in Table 2. It was observed that the height was significantly lower in Group 2 (127.5 ± 13.5 cm, height Z-score −1.7 ± 1.7) versus Group 1 (133.5 ± 17.7 cm, height Z-score −0.1 ± 1.4). The BMI in the two study groups was comparable: 18.9 ± 3.7 kg/m2 in Group 1 versus 19.4 ± 4.6 kg/m2 in Group 2.
| Parameter | Group 1 (n=20) | Group 2 (n=18) |
|---|---|---|
| Symptoms (%) | ||
| Fatigue | 3 (15) | 3 (16.6) |
| Poor growth | 7 (35) | 5 (27.7) |
| Weight gain | 8 (40) | 8 (44.4) |
| Constipation | 7 (35) | 8 (44.4) |
| Goiter (%) | 9 (45) | 11 (61.1) |
| Family history (%) | 9 (45) | 8 (44.4) |
| TSH level (median) (µIU /mL) |
22 | 54 |
| Free T4 level (median) (ng/dL) |
1.03 | 0.9 |
| Antithyroid peroxidase antibody titer (median) (IU/mL) | 234 | 234 |
| Anti-thyroglobulin antibody titer (median) (IU/mL) | 234 | 79.9 |
TSH: Thyroid-stimulating hormone
| Parameter (mean value) | Group 1 (n=20), four males | Group 2 (n=18), eight males | ||
|---|---|---|---|---|
| Baseline | Endline | Baseline | Endline | |
| Age | 9.7±2.9 | 10.8±2.8 | 10.7±2.4 | 11.9±2.0 |
| BA* | 9.8±2.7 | 11.1±2.6 | 7.9±2.5 | 11.6±1.7 |
| Height (in cm) | 133.5±17.7 | 139.9±17.6 | 127.5±13.5 | 135.0±11.0 |
| Height Z-score*,a | –0.1±1.4 | –0.1±1.1 | –1.7±1.7 | –1.3±1.3 |
| Body mass index (in kg/m2) | 18.9±3.7 | 17.9±7.9 | 19.4±4.6 | 17.8±7.8 |
| BMI Z-score | 0.6±1.3 | 0.1±1.1 | 0.6±1.1 | 0.0±0.8 |
| Pre-pubertal: pubertala | 15:5 | 11:9 | 14:4 | 7:11 |
| Predicted adult height | 158.5±12.3 | 157±12.5 | 158.5±13.3 | 150.0±13.0 |
| PAH Z-scorea | –0.5±1.7 | –0.7±1.6 | –1.1±1.6 | –2.2±1.4 |
| Target height Z-score | –1.1±1.1 (152±6.1) | –0.4±1.7 (158.0±9.6) | ||
The recruited study subjects were managed and followed up in the thyroid clinic. During the study period, 80 and 72 follow-up visits were observed in Groups 1 and 2, respectively. The number of visits with elevation in TSH (>10 μIU/ml) and low free T4 (<0.9 ng/dL) was 21.2% and 10% in Group 1 and 20.8% and 9.7% in Group 2, respectively (P > 0.05). The median TSH and free T4 on follow-up were 3.3 μIU/ml and 2.9 μIU/ml and 1.6 ng/dl and 1.5 ng/dl, respectively, in Groups 1 and 2. At the end of 1 year of follow-up, we observed that height increased from 133.5 ± 17.7 cm to 139.9 ± 17.6 cm and 127.5 ± 13.5 cm to 135.0 ± 11.0cm, in Groups 1 and 2, respectively. This corresponds to a change in height Z-score from −0.1 ± 1.4 to −0.1 ± 1.1 and −1.7 ± 1.7 to −1.3 ± 1.3 in Groups 1 and 2, respectively. The parallel changes in BMI Z-score were: 0.6 ± 1.3–0.1 ± 1.1 in Group 1 and 0.6 ± 1.1–0.0 ± 0.8 in Group 2. Growth velocity in subjects in two groups showed growth velocity above 50th percentile in nine out of 20 subjects in Group 1 (45%) versus 11 out of 18 subjects in Group 2 (61.1%).
We calculated the BA: CA ratio (BA: CA) in Groups 1 and 2. We observed that the BA: CA ratio changed from 1.0 ± 0.1 to 1.0 ± 0.1 in Group 1 (P > 0.05) versus 0.7 ± 0.2 to 0.9 ± 0.1 in Group 2 (P < 0.05) [Figure 1]. The number of children who were pre-pubertal: pubertal changed from 15:5 to 11:9 in Group 1 and 14:4 to 7:11 in Group 2. We also observed that 9 subjects (45%) in Group 1 and 11 subjects in Group 2 (61%) had a progress in puberty assessed by Tanner’s staging. To study the impact of therapy on growth potential of the study subjects, we calculated PAH using the Bayley-Pinneau tables. For Group 1, baseline PAH Z score was −0.5 ± 1.7 and endpoint PAH Z score was −0.7 ± 1.6 versus a target height Z score of −1.1 ± 1.1 (P > 0.05). In study subjects in Group 2, the baseline PAH Z score −1.1 ± 1.6 and endpoint PAH Z score −2.2 ± 1.4 versus a target height Z-score of −0.4 ± 1.7 (P < 0.05) [depicted in Figure 2]. The median PAH Z-score was −0.3 (range: –5.6–+1.7) and −0.4 (range: –5.2–+2.2) at baseline and endpoint in Group 1; the median PAH Z-score was −0.6 (range: –4.6–+1.3) and −2.1 (range: –4.4–0.7) at baseline and endpoint in Group 2, respectively.
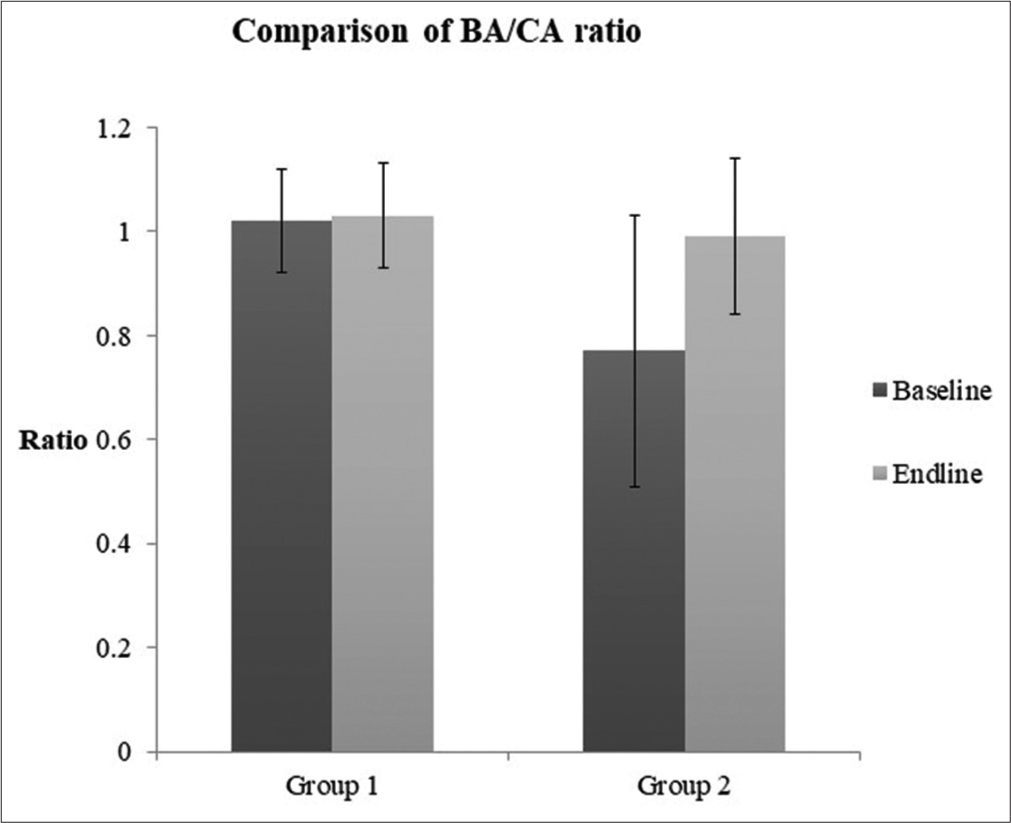
- Comparison of bone age: Chronological age ratio in study Groups I and II.
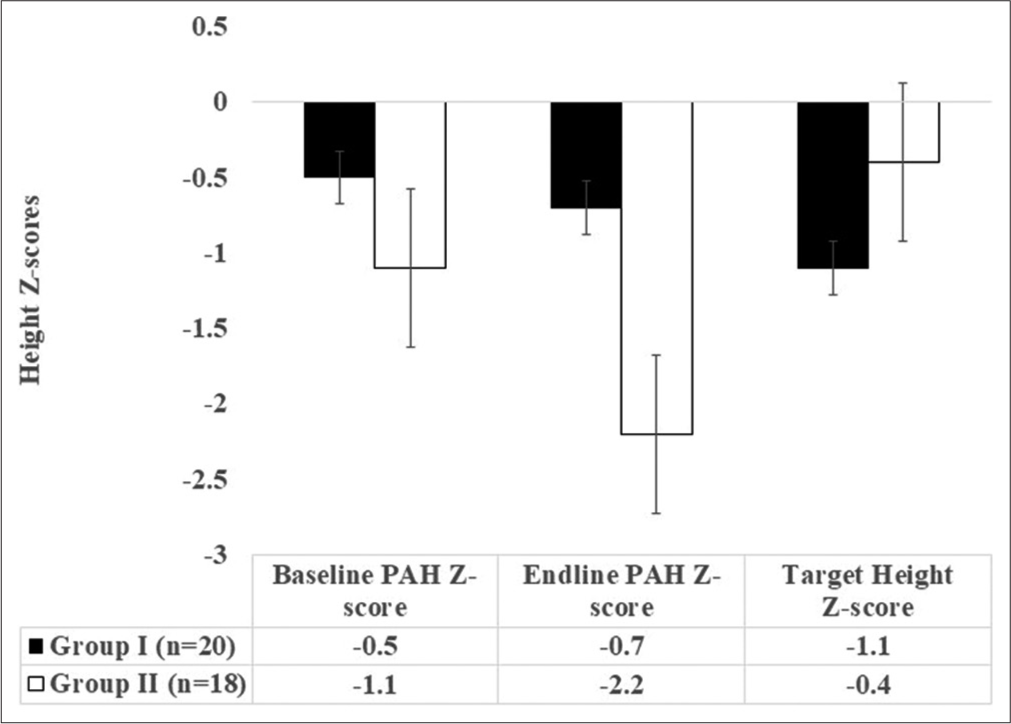
- Comparison of predicted adult height Z-scores baseline and endline versus target height in the two study groups.
To further elucidate the tempo of growth progression, we calculated pace in the two groups. We observed that the pace was 3.5 ± 2.6 in Group 2 versus 1.4 ± 1.6 in Group 1 (P < 0.05). To further elucidate the relationship between pace and final height, we attempted to correlate analysis between the two. We observed a moderate negative correlation (r = –0.5, P = 0.02), between pace and endline PAH Z-scores.
All the study subjects continued to be followed up in the thyroid clinic. Those with PAH <150 cm (in girls) and <160 cm (in boys) were counseled on experimental growth promoting options such as growth hormone therapy and gonadotropin analog therapy.
DISCUSSION
To the best of our knowledge, this is the first Indian study which demonstrates that delayed diagnosis of hypothyroidism can result in the loss of permanent height potential in spite of thyroxine supplementation and hence early diagnosis is important. In our 1 year follow-up study on 38 children with HT, we have demonstrated a good catch-up in short-term height Z-scores and reduction in BMI. We also observed a relative rapid skeletal maturity advancement in subjects with delayed BA at initiation of therapy. This has resulted in a significant loss of height potential and reduction in predicted final height. A previous Indian study described that 6 months of thyroxine therapy lead to skeletal maturity advancement by 11 months.[10] Out of 29 children with HT, 21 and eight children had delayed BA at baseline and endline.[10] However, the authors have not described the impact on height potential based on these observations. A long-term follow-up study on 18 girls and six boys with HT showed that final height Z-scores were lower than initial pre-treatment height Z scores and target height Z-scores.[7] After 18 months of therapy, they found that the skeletal maturation exceeded the statural growth. At maturity, all the children stood 2 SDs below the normal adult stature. Another study on four children from the Netherlands demonstrated a remarkable short-term catch-up with thyroxine and permanent loss of final height potential.[5] In contrast to our observations, a retrospective study on 21 children with juvenile hypothyroidism from Indianapolis showed that neither time to euthyroidism nor growth promoting therapy impacts the final height in children with juvenile autoimmune hypothyroidism.[21]
We consider three possibilities for the loss of height potential in these children. First, overtreatment with thyroxine to improve clinical symptomatology, especially, in those with delayed BA should be avoided.[19,20,23] The impact of thyroid hormones on advancing BA has been explored previously in hyperthyroidism.[24] Second, there is diminished growth due to the effect of prolonged hypothyroidism. Duration of hypothyroidism (reflected in normal skeletal maturation in Group 1 and significant delayed BA in Group 2) and undue rapid catch-up resulted in change in BA: CA ratio from 1.02 ± 0.1 to 1.03 ± 0.1 in Group 1 (P > 0.05) versus 0.7 ± 0.1 to 0.9 ± 0.1 in Group 2 (P < 0.05). The advancement in BA resulted in lesser years of growth and reduced predicted height versus the genetic potential (reflected in target height). Third, rapid sexual maturation with thyroxine result in sex steroids advancing skeletal maturation. We also observed that 45% in Group 1 and 61% in Group 2 had an advancement in SMR staging. To annul this loss of height potential, one could consider gonadotropin analog therapy and growth hormone therapy, which is beyond the scope of our study.[9]
There are two aspects that we would like to clarify in our study. PAH was used for predicting the final height at maturity for children based on BA and residual growth potential. However, one should follow-up these children till maturity to see if they attain the PAH. Second, we used Greulich-Pyle atlas for BA estimation as it has been previously reported to estimate BA accurately in children with hypothyroidism.[25]
Our study is not without limitations. The follow-up period of 1 year is inadequate to study long-term growth, especially as thyroid hormone has multiple actions and physiologically essential adaptations may take priority at the start of thyroxine replacement rather than growth. Our subjects should be followed up at least for a period of 3 years to analyze the impact on long-term growth. The free T4 levels in Group 2 were marginally lower than Group 1. Hence, severity of hypothyroidism along with duration of hypothyroidism may also contribute to the observations in catch-up growth. Thus, there is a need for further studies on children with primary hypothyroidism on long-term auxological outcomes.
CONCLUSION
Thus, we conclude that thyroxine treatment in children with juvenile-acquired hypothyroidism leads to good catch-up growth and sexual maturation. Children who present late with HT may initially have rapid catch-up growth with thyroxine, but finally have permanent loss of height potential and hence they may need to be identified early and treated less aggressively.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Long-term follow-up and outcomes of autoimmune thyroiditis in childhood. Front Endocrinol (Lausanne). 2020;11:309.
- [CrossRef] [PubMed] [Google Scholar]
- Juvenile hypothyroidism: A clinical perspective from Eastern India. Indian J Endocrin Metab. 2020;24:260-4.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic autoimmune thyroiditis in children and adolescents: At presentation and during long-term follow-up. Arch Dis Child. 2009;94:33.
- [CrossRef] [PubMed] [Google Scholar]
- Determinants of levothyroxine dose required to achieve euthyroidism in pediatric population-a hospital-based prospective follow-up study. Eur J Pediatr. 2017;176:1027-33.
- [CrossRef] [PubMed] [Google Scholar]
- Catch-up growth after prolonged hypothyroidism. Eur J Pediatr. 1996;155:362-7.
- [CrossRef] [PubMed] [Google Scholar]
- Head circumference, height, bone age and weight in 103 children with congenital hypothyroidism before and during thyroid hormone replacement. Helv Paediatr Acta. 1985;40:305-16.
- [Google Scholar]
- Long-term growth in juvenile acquired hypothyroidism: The failure to achieve normal adult stature. N Engl J Med. 1988;318:599-602.
- [CrossRef] [PubMed] [Google Scholar]
- Growth prognosis and growth after menarche in primary hypothyroidism. Arch Dis Child. 1991;66:838-40.
- [CrossRef] [PubMed] [Google Scholar]
- Use of growth hormone and gonadotropin releasing hormone agonist in addition to L-thyroxine to attain normal adult height in two patients with severe Hashimoto's thyroiditis. J Pediatr Endocrinol Metab. 2005;18:515-21.
- [CrossRef] [PubMed] [Google Scholar]
- Skeletal manifestations of juvenile hypothyroidism and the impact of treatment on skeletal system. Indian J Endocr Metab. 2013;17:181-3.
- [CrossRef] [PubMed] [Google Scholar]
- Hashimoto's thyroiditis in South Indian centre. Indian J Pediatr. 2016;83:1227-31.
- [CrossRef] [PubMed] [Google Scholar]
- Subclinical hypothyroidism in children. Indian Pediatr. 2014;51:889-95.
- [CrossRef] [PubMed] [Google Scholar]
- Autoimmune thyroid disease in childhood: A study of children and their families. Indian Pediatr. 1999;36:659-68.
- [Google Scholar]
- Revised IAP growth charts for height, weight and body mass index for 5-to 18-year-old Indian children. Indian Pediatr. 2015;52:47-55.
- [CrossRef] [PubMed] [Google Scholar]
- Height velocity percentiles in Indian children aged 5-17 years. Indian Pediatr. 2019;56:23-8.
- [CrossRef] [PubMed] [Google Scholar]
- Radiographic Atlas of Skeletal Development of the Hand and Wrist (2nd ed). Stanford, CA: Stanford University Press; 1959. p. :125-83.
- [Google Scholar]
- Tables for predicting adult height from skeletal age: Revised for use with the Greulich-pyle hand standards. J Pediatr. 1952;40:423-41.
- [CrossRef] [Google Scholar]
- Reassessment of the daily dose of oral thyroxine for replacement therapy in hypothyroid children. J Pediatr. 1977;90:291-7.
- [CrossRef] [Google Scholar]
- Evaluation of sodium L-thyroxine (T4) requirement in replacement therapy of hypothyroidism. J Pediatr. 1977;90:298-301.
- [CrossRef] [Google Scholar]
- Does clinical management impact height potential in children with severe acquired hypothyroidism? J Pediatr Endocrinol Metab. 2011;24:893-6.
- [CrossRef] [Google Scholar]
- The use of bone age in clinical practice-part 1. Horm Res Paediatr. 2011;76:1-9.
- [CrossRef] [PubMed] [Google Scholar]
- Attainment of normal height in severe juvenile hypothyroidism. Arch Dis Child. 1994;70:429-31.
- [CrossRef] [PubMed] [Google Scholar]
- Neonatal thyrotoxicosis: Intellectual impairment and craniosynostosis in later years. J Pediatr. 1980;97:257-9.
- [CrossRef] [Google Scholar]
- Skeletal maturation during thyroxine treatment in children with congenital hypothyroidism. Acta Paediatr. 1994;83:618-22.
- [CrossRef] [PubMed] [Google Scholar]






