Translate this page into:
Efficacy of the MiniMed™ 670G hybrid closed loop system in managing postprandial glucose excursion with high protein high fat foods in children and adolescents under free-living conditions

*Corresponding author: Mary B. Abraham, PhD, Children’s Diabetes Centre, Telethon Kids Institute, The University of Western Australia, Perth, Australia. mary.abraham@health.wa.gov.au
-
Received: ,
Accepted: ,
How to cite this article: Lim RJ, Abraham MB, Nicholls R, Fournier PA, Harray AJ. Efficacy of the MiniMed™ 670G hybrid closed loop system in managing postprandial glucose excursion with high protein high fat foods in children and adolescents under free-living conditions. J Pediatr Endocrinol Diabetes 2023;3:63-70.
Abstract
Objectives:
High protein high fat (HPHF) meals are considered “difficult” foods because they can cause prolonged hyperglycemia after ingestion. The potential of hybrid closed loop therapy in managing postprandial glucose excursions with these difficult foods remains unknown. This pilot study aimed to explore the impact of manual mode in standard insulin pump therapy and auto mode with hybrid closed loop pump therapy in managing glucose excursions caused by HPHF foods and to obtain feedback from families about each mode.
Material and Methods:
Children and adolescents (8–18 years) with type 1 diabetes and using the MiniMed™ 670G were recruited to a free-living randomized cross-over study. Participants consumed a standardized lasagne or pizza meal two nights a week for 4 weeks while in auto mode and manual mode. Postprandial continuous glucose monitoring data were collected for 7 h post-meal. The primary outcomes were mean postprandial net incremental area under the glucose × time curve. User experiences were collected during end-of-study interviews administered to parents.
Results:
Postprandial excursions from 38 meals in seven participants were analyzed. There were no significant differences between auto mode and manual mode for the mean net incremental area under the glucose × time curve, irrespective of meal type. Semi-structured end-of-study interviews revealed that five of seven families felt more confident eating HPHF meals in auto mode.
Conclusion:
Although most families felt confident with auto mode for postprandial HPHF excursions, this was not reflected in the postprandial glucose levels.
Keywords
Closed loop therapy
glycemic control
high protein high fat meals
postprandial glucose excursions
type 1 diabetes
INTRODUCTION
In Australia, 41% of the daily energy intake of children and adolescents comes from energy-dense nutrient-poor (or discretionary) foods and beverages,[1] often high in protein and fat (HPHF). Research investigating the impact of dietary protein and fat on postprandial glucose excursions has shown that HPHF meals can result in late, prolonged glucose excursions that can last for up to 10–12 h after eating in individuals with type 1 diabetes (T1D).[2-5] The peak increase in glucose readings has been detected at approximately 6-h post-meal.[3] For HPHF foods that cause postprandial hyperglycemia, the current guidelines suggest a conservative starting point of 15–20% extra mealtime insulin dose when using multiple daily injections, and a combination bolus for standard insulin pump therapy.[6] However, there are no evidence-based recommendations to guide the use of hybrid closed loop therapy for HPHF foods. The potential of hybrid closed loop therapy in managing postprandial glucose excursions with these difficult foods remains unknown.[7]
The automated basal insulin delivery in closed loop systems and the lack of combination boluses highlight the need to explore how automated insulin delivery in closed loop therapy compares to standard insulin pump therapy in managing postprandial hyperglycemia after consuming HPHF meals. Chan et al.[8] compared the use of auto mode versus manual mode with food in children in a camp setting and found that children had more in-target glucose levels with auto mode in a diabetes camp setting. However, to the best of our knowledge, no study has investigated how the auto mode feature responds specifically to HPHF meals in children. Hence, our pilot study aimed to explore the impact of HPHF foods on glucose excursions in children using the Medtronic (Minneapolis, Minnesota, USA) 670G hybrid closed loop system (auto mode), compared to manual mode, under free-living conditions. The lived experiences of families managing postprandial excursions with HPHF meals with both modes of insulin delivery were also explored.
MATERIAL AND METHODS
Design
This prospective, randomized, and cross-over pilot study was conducted at a tertiary pediatric diabetes hospital in Perth, Western Australia. Participants were randomized to HPHF meals in manual mode and auto mode with the free-living study lasting 28 days for each participant, with 4-study days under controlled conditions in each arm [Figure 1]. The manual mode of the Medtronic MiniMed™ 670G replicates the operation of the more widely used standard insulin pump therapy, whereas the auto mode utilizes real-time continuous glucose monitoring (CGM) data to modulate insulin delivery. In both modes, the user enters the carbohydrate (CHO) content of the meal to provide the meal bolus.
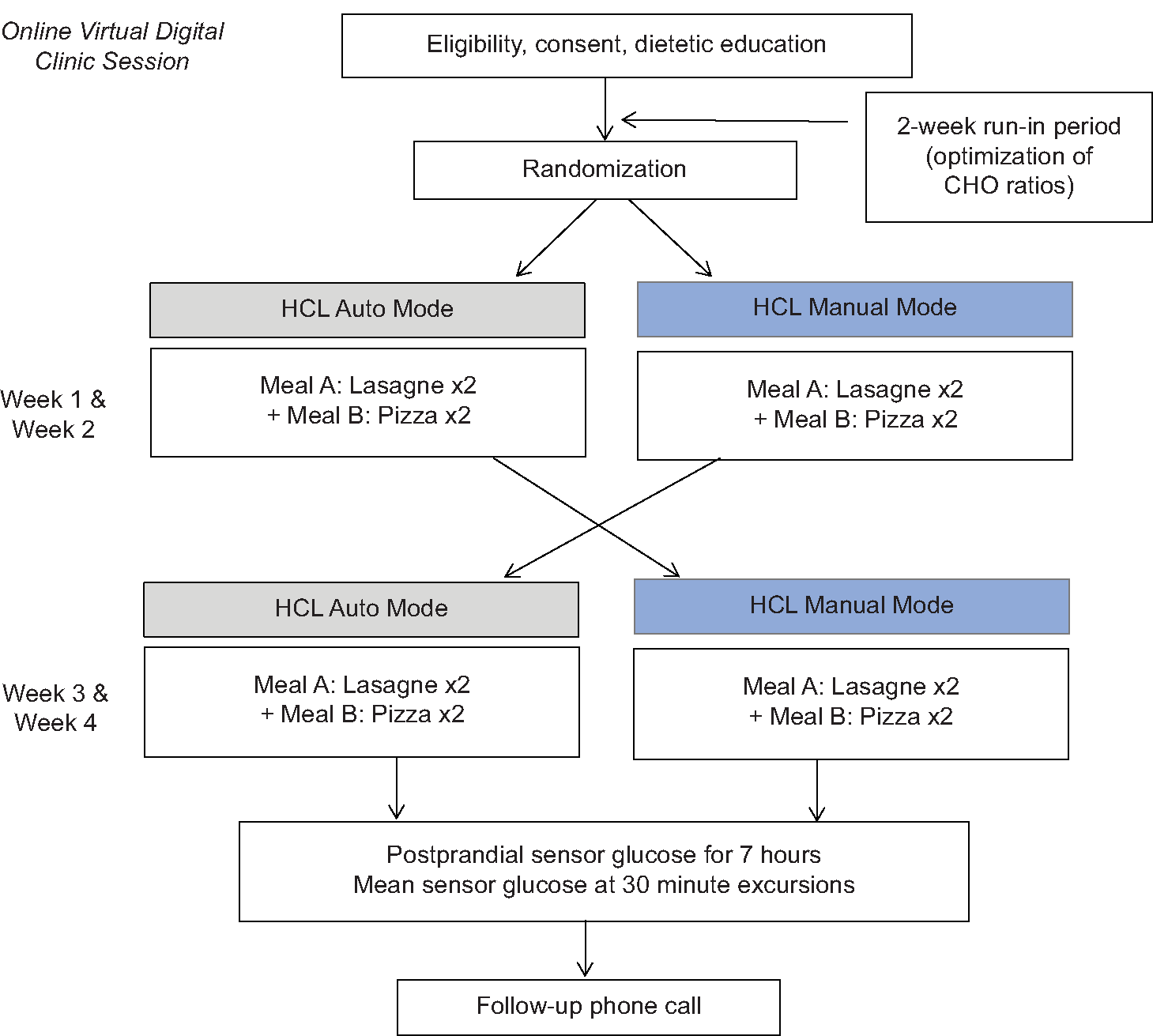
- Overview of study design
The study was approved by the Child and Adolescent Health Service Ethics Committee. Before data collection, informed consent was obtained from the parent of the child with T1D, and assent of adolescents was obtained if deemed mature in line with the current ethics protocol.
Participants
Participants in this study were aged 8–18 years; had T1D for at least 1 year; glycated hemoglobin (HbA1c) <8.5%; using a Medtronic MiniMed™ 670G insulin pump and Guardian G3 CGM sensor and using auto mode for more than 1 month. Participants were excluded if they had celiac disease (as the test meals contained gluten) and/or gastroparesis.
Methods
After a 2-week run in the period following dietetic education and optimization of insulin pump settings with an endocrinologist, participants were randomized to one of two sequences, either auto mode (hybrid closed loop therapy) or manual mode (standard pump therapy) first. Randomization was conducted independently, using randomly permuted blocks with random block sizes on the available software www.randomization.com. Participants began the study in their allocated modes and then switched modes after 2 weeks with test meals consumed during each mode [Figure 1a and b].
Test meals
Two standardized HPHF test meals were used in the intervention, McCain Beef Lasagne™ (20 g fat; 20 g protein; 80 g CHOs; and 2470 kJ/serve) and McCain Margherita Pizza™ (30.6 g fat; 32 g protein; 70 g CHOs; and 2900 kJ/serve) in 400 g and 250 g serves, respectively. These test meals were used as lasagne and pizza and were identified as “difficult meals” by families living with T1D attending the Perth Children’s Hospital Diabetes Clinic (Binkowski S, unpublished data). The specific brand was selected as it provided a list of ingredients and provided the proportion of CHO, fat, and protein. Frozen meals were chosen as they were widely available in mainstream supermarkets throughout the state of Western Australia.
Run-in period
Participants were using auto mode as their standard management. At the beginning of the study, all participants and their parents were re-educated through a telephone consultation with an Accredited Practicing Dietitian on how to count CHOs, read nutrition information panels, and how to manage HPHF meals in manual mode using the ISPAD guidelines.[6] All families were free to choose their preferred pre-meal bolus pattern in manual mode. This was followed by a 2-week run-in period whereby a study doctor aimed to optimize the participant’s glucose levels by reviewing and adjusting basal insulin, insulin-to-CHO ratios, and insulin sensitivity factors, as required. This was done to improve the likelihood of the participant being within the target glucose range before consumption of the test meal. Pump settings were kept constant across study days for all participants.
Data collection
On each of the participants’ chosen study days, they were advised to follow their usual food, physical activity, and insulin regimen until 3 pm. Participants were instructed on how to complete a written food diary, including the time and amount of each test meal consumed on each study day, and the amount of pre-meal insulin during the allocated study phase. Participants recorded their food and beverage intake and physical activity to assist them in matching these variables across the study days. Participants were asked to fast and avoid any vigorous physical activity for a minimum of 3 h before each test meal and have sensor glucose reading between 70 and 180 mg/dL. Study days were deferred if these prerequisites were not met. All participants were encouraged to “eat to appetite” for the first margherita pizza and beef lasagne test meals, and then match the amount of food eaten for the remaining study days.
Each participant was required to consume a total of four test meals during 2 weeks in each mode (e.g., two lasagne and two pizza meals in manual mode). They could consume two different test meal types (pizza and lasagne) within the same week. All participants were asked to consume each test meal within 20 min. No other food or drink (except water) could be consumed with each test meal and for the proceeding 7 h after. Participants were asked to avoid any vigorous physical activity during this monitoring period. A 7-h follow-up period was chosen to capture the peak glucose excursion.
On auto mode study days, participants were advised to administer insulin corrections as directed by the system. When using auto mode, correction boluses are recommended by the system if sensor glucose is above 150 mg/dL. The participant is given the option to make a correction if reflected in their standard care. When participants were using manual mode on a study day, they were required to manually exit auto mode and change to manual mode from 3 pm onwards, which was approximately 3 h before the test meal. When using manual mode, participants were asked to refrain from making any insulin corrections during the 7-h postprandial fasting period if their glucose levels were below 270 mg/dL to demonstrate the effects of the insulin boluses in manual mode. However, participants were given the option to make an insulin correction if their glucose level was above 270 mg/dL to reflect the usual practice of most families. After the 7-h postprandial period, participants could switch back to auto mode. Participants were advised to follow standard hypoglycemia management protocol as per the education provided in the clinic for either the standard pump therapy or hybrid closed loop therapy.
Qualitative interview
Within 1 week of completing the study, all participants and their parents were contacted for a semi-structured telephone interview containing open-ended questions to gather information on their experiences. The questions around the challenges of HPHF and difficult foods were explored at the end of the study interviews.
Outcome measures
Postprandial glucose excursions were collected at 30-minute intervals from baseline to 420 min (7 h) after each test meal. This information was used to calculate the net incremental area under the glucose curve, which was the area between the sensor glucose level (SGL) × time curve and pre-meal baseline, where the areas above baseline are positive and below baseline are negative. Other outcome measures included in the study; absolute incremental area under the glucose curve, area under the curve, time in range, peak SGL, time to peak SGL, and the difference between peak SGL and baseline SGL. Hypoglycemic events are defined as SGL <70 mg/dL.
Statistical analysis
A linear mixed model was carried out to analyze the difference in mean glucose excursions and net incremental and absolute areas under the sensor glucose curve for each participant, with mode type as the predictor and a random intercept included for the participant. This approach was chosen to account for the repeated measurements on the same participant. The following variables were analyzed: area under the curve, time in range, peak SGL, and the difference between peak SGL and baseline SGL. The difference between auto mode and manual mode for each meal type was calculated but not between different meal types. Data analyses were included if either pizza or lasagne was consumed in both auto mode and manual mode by a participant.
Statistical analyses were performed using STATA statistical software (version 16.1), with P < 0.05 considered statistically significant. Qualitative data of observational and behavioral findings from end-of-study interviews were reported.
RESULTS
Data from 21 pizza meals and 17 lasagne meals were analyzed from seven participants (three males and four females). The mean ± standard deviation for the age of participants was 12.4 ± 2.9 years, duration of diabetes 4.5 ± 2.6 years, HbA1c 7.3 ± 0.4%, and auto mode use 7.8 ± 3.4 months. Participants spent 82.5 ± 7.9% of their time in auto mode before the start of the study.
Three families chose to use the dual wave pattern when in manual mode during the study. Two participants reported regular use of this insulin delivery pattern at home to manage HPHF meals before the study while one participant chose the dual wave option following the dietetic re-education sessions. Four participants, after being re-educated and encouraged to use the dual wave bolus option during the free-living study, chose not to adopt this insulin delivery mode at home for the HPHF meals and used their preferred standard pre-bolus insulin delivery pattern. Reasons for not using dual wave in manual mode included a lack of confidence, unwillingness, or previous pre-meal bolusing pattern that works for them when in manual mode.
All participants reported completing each test meal on each study day. A total of 61 meals were ingested including 34 pizza meals and 27 lasagne meals by 10 participants. Three participants withdrew from the study due to pump technical issues (n = 2) and disliking the meals (n = 1). The meals consumed by the three participants before withdrawal were not included in the final analysis as they did not consume the test meals in both modes. Thirteen pizza meals and 10 lasagne meals were not included in the final analyses for the following reasons: protocol deviations (n = 11), hypoglycemic events (n = 9), and technical issues (n = 3). Examples of protocol deviations included; not switching to manual mode 3 h before the study meal and administering insulin corrections when SGL was below 270 mg/dL. All pizza-fed participants in auto mode complied with the protocol, whereas five of seven participants complied with the manual mode. Five of seven lasagne-fed participants in auto mode complied with the protocol and five of seven participants in manual mode complied with the protocol. The data from participants who did not comply were not used in the analyses, thus explaining the different number of participants and meals between insulin delivery modes.
Effect of auto mode and manual mode on sensor glucose responses after test meals
Baseline SGL for manual mode was 135 ± 23 mg/dL (pizza) and 159 ± 49 mg/dL (lasagne). For auto mode, the baseline SGL was 115 ± 36 mg/dL (pizza) and 124 ± 18 mg/dL (lasagne). Participants consumed a mean intake of 293 g of pizza and 400 g of lasagne. Three families used the dual wave pattern to bolus insulin when in manual mode during the study for four meals. For the three participants who used the dual wave pattern on four separate meals, their manual mode pre-meal bolus patterns included: 60/40% split over 3 h (n = 2); 50% upfront/50% over an hour (n = 1), and a square wave over 3 h (n = 1). Two out of seven participants split their meal insulin bolus in auto mode. One participant administered a split bolus for two (one pizza and one lasagne) of the four meals, and the other participant administered a split bolus for all their meals. There was no statistically significant difference between auto mode and manual mode for the following variables: Net incremental area under the glucose × time curve; absolute incremental area under the glucose × time curve; area under the glucose × time curve; time in range; peak SGL; time to peak SGL; and the difference between peak SGL and baseline SGL [Table 1a and b].
| Pizza (n=21) | |||||
|---|---|---|---|---|---|
| Auto mode | Manual mode | Difference | 95% CI | P-value | |
| Net iAUC (mg.hour/dL) | 311.4±210.6 | 248.4±257.4 | −63.0±46.8 | −417.6, 291.6 | 0.67 |
| Absolute iAUC (mg.hour/dL) | 406.8±142.2 | 432.0±214.2 | 25.2±72.0 | −241.2, 293.4 | 0.79 |
| AUC (mg.h/dL) | 1168.2±217.8 | 1227.6±252 | 59.4±34.2 | −297.0, 415.8 | 0.69 |
| Time in range (%) | 70.4±23.4 | 52.5±27.8 | −17.9±4.4 | −0.6, 0.2 | 0.29 |
| Peak SGL (mg/dL) | 230.4±43.2 | 248.4±46.8 | 16.2±1.8 | −54.0, 88.2 | 0.57 |
| Time to peak SGL (minutes) | 277.0±98.0 | 301.0±93.0 | 24.0±5.0 | −1240.0, 172.0 | 0.70 |
| Difference between peak SGL and baseline SGL (mg/dL) | 117±48.6 | 115.2±45 | −1.8±3.6 | −73.8, 70.2 | 0.94 |
Values are presented as mean±standard deviation, P-value calculated using mixed models. iAUC: Incremental area under the curve, AUC: Area under the curve, SGL: Sensor glucose levels, CI: Confidence interval. Difference=Manual mode–Auto mode
| Lasagne (n=17) | |||||
|---|---|---|---|---|---|
| Auto mode | Manual mode | Difference | 95% CI | P-value | |
| Net iAUC (mg.hour/dL) | 162.0±77.4 | −52.2±196.2 | −214.2±118.8 | −475.2, 46.8 | 0.09 |
| Absolute iAUC (mg.hour/dL) | 293.4±90.0 | 270.0±95.4 | −23.4±5.4 | −160.2, 111.6 | 0.69 |
| AUC (mg.h/dL) | 1087.2±189.0 | 1117.8±264.6 | 30.6±77.4 | −262.8, 322.2 | 0.79 |
| Time in range (%) | 73.8±24.9 | 72.4±26.6 | −1.4±1.6 | −0.3, 0.3 | 0.91 |
| Peak SGL (mg/dL) | 212.4±28.8 | 199.8±54.0 | −12.6±25.2 | −50.4, 25.2 | 0.40 |
| Time to peak SGL (minutes) | 163.0±110.0 | 310.0±151.0 | 147.0±41.0 | −65.0, 359.0 | 0.91 |
| Difference between peak SGL and baseline SGL (mg/dL) | 88.2±19.8 | 41.4±48.6 | −46.8±28.8 | −104.4, 0.9 | 0.08 |
Values are presented as mean±standard deviation, P-value calculated using mixed models. iAUC: Incremental area under the curve, AUC: Area under the curve, SGL: Sensor glucose levels, CI: Confidence interval. Difference=Manual mode–Auto mode
Additional insulin boluses
In auto mode, insulin corrections were administered as directed per the system. Out of the 21 auto mode meals, 12 insulin corrections were given (n = 5 out of 7). Of 17 manual mode meals, three insulin corrections were given (n = 3 out of 7). After the 7-hours postprandial period for manual mode meals, all families switched back to auto mode.
Number of hypoglycemic events
A total of nine hypoglycemic events (SGL <70 mg/dL) were experienced by five participants and these events occurred within 2 h of consuming the meal. Five hypoglycemic events were associated with manual mode and four events were with auto mode. One participant experienced four out of the nine hypoglycemic events, one participant experienced two events and the remaining three participants had one hypoglycemic event each. These data were not included in the final analysis.
No episodes of severe hypoglycemia were experienced in the study by participants.
Lived experiences of manual versus auto mode
Qualitative findings on the families lived experiences of using both modes were documented in the end of the study interview. Five families noted that they preferred auto mode when eating difficult foods, one family preferred manual mode and one family noted similar responses with both modes. Reasons for a preference toward auto mode included a reduction in guesswork and burden. Responses from the parents of participants to our three main questions are presented [Table 2].
| Did you find it challenging to control blood glucose levels after eating pasta and pizza before the study? | “We find pizza a difficult food where his blood glucose levels would spike in the late evening. We don’t really have an issue with pasta.” - Parents of 8-year-old boy |
| “Yes, pizza and pasta were challenging foods for us. After eating these foods, her blood glucose levels would remain high for around five to 7 hours after eating and it would be after 2 am that they would peak. We didn’t realize that it was the high fat and high protein content that caused the highs, we thought her blood levels were playing up.” - Parent of 9-year-old girl | |
| “After eating pizza and pasta, we find that his levels are always high, delayed and long and it would remain high the whole night if we did not tightly manage it. His highs were definitely not in range. It was a fight to bring his levels back down. There was no gentle way of doing it. He crashed too fast and there was no slowdown.” - Parent of 9-year-old boy | |
| Do you find any other types of food difficult? | “Yes, garlic bread. No matter the brand or where we get it from.”-Parent of 8-year-old boy |
| “Rice and fish and chips.”- Parent of 13-year-old girl | |
| “Take away foods are difficult food for her.” - Parent of 14-year-old girl | |
| “Rice is a very difficult food for us. His levels will spike around 6–8 h after eating. We only try to eat brown basmati rice now.” - Parent of 16-year-old boy | |
| “Just pizza and pasta in general.” - Parent of 9-year-old girl | |
| “Fresh salmon, Weet-Bix, even the low GI range and all cereals are difficult foods for us. We have been on a low carb breakfast for 1–2 years now.”- Parent of 9-year-old boy | |
| Which mode do you prefer, and do you feel like one Mode dealt with the high fat and high protein foods better? | “I found no difference between auto mode and manual mode for pasta but when he ate the pizza, I found he went higher in manual mode and not so high when he was in auto mode.”-Parent of 8-year-old boy |
| “I felt more control when in auto mode. Manual mode was more challenging.” - Parent of 13-year-old girl | |
| “Auto mode was significantly better.” - Parent of 14-year-old girl | |
| “I prefer auto mode because it gives me a sense of security and reassurance. I have more confidence when in auto mode, especially overnight.” - Parent of 16-year-old boy | |
| “I am surprised that [my daughter]had similar traces with both manual mode and auto mode.”- Parent of 11-year-old girl | |
| “Her levels went high after a couple of hours in auto mode. I felt like the dual wave mode in manual mode was definitely better than auto mode.” - Parent of 9-year-old girl | |
| “Auto mode was perfect. I felt more in control and liked how the pump can stop or give more background insulin based on his glucose levels. Auto mode dealt with these foods better and there was less manual intervention.” - Parent of 9-year-old boy |
DISCUSSION
In this pilot study, there were no appreciable differences in postprandial glucose levels between standard insulin pump therapy and automated insulin therapy after the consumption of HPHF meals. No significant difference was noted between auto mode and manual mode for either meal type. Many previous studies have indicated the benefits of a closed loop on glycemic outcomes;[9-11] However, no observational or interventional trials have considered the effect of HPHF meals on postprandial glycemia. These findings indicate that it is important for future studies to consider the effect of food, including HPHF foods on postprandial glycemia, especially with newer and more advanced automated systems.
Despite the lack of significant difference between auto mode and manual mode on postprandial glucose levels, the qualitative semi-structured end-of-study interviews revealed that six of seven families felt more confident consuming the HPHF meals in auto mode. This may be partly related to how they managed HPHF meals in manual mode. The current recommendations are to use a dual wave bolus in manual mode to help manage postprandial glycemia after HPHF meals; however, it is interesting to note that participants did not use the dual wave bolus function as anticipated because of a lack of confidence and unwillingness. All families were re-educated to use dual wave in manual mode and we expected a higher usage of dual wave. This may have influenced the postprandial glycemic response in manual mode. A possible reason for participants’ preference toward auto mode may be the reduction in guesswork as insulin delivery is modulated by the real-time SGL with less user intervention, resulting in a possible reduction in responsibility and burden on the family.[12,13] It is important to highlight that our findings should be interpreted with caution given; all participants recruited to the study were regular users of auto mode, thus entailing that our participants preferred the use of auto mode. It remains unknown if individuals on standard insulin pump therapy with tight glycemic control would have preferred the auto mode. Given the less-than-optimal performance of the auto mode for some HPHF meals, it is possible that they would prefer the manual over the auto mode because the manual mode provides them with the option of more closely controlling their glucose management.
The high glucose levels 7 h after the pizza meal in almost all participants in both modes are a finding consistent with those of many earlier studies reporting that HPHF meals cause large and delayed postprandial glucose excursions in individuals with T1D.[2-5] More importantly, our finding suggests that the auto mode algorithm performs suboptimally at normalizing glucose level in response to some HPHF meals (e.g., pizza).
The 670G system is less robust in correcting postprandial hyperglycemia as it was designed with a conservative algorithm with a high glucose target to safeguard against hypoglycemia[14] and was not designed to address and correct postprandial rises. It remains to be determined whether the newer systems (Medtronic 780G with SmartGuard, Tandem Diabetes Care t: slim X2 insulin pump with Control-IQ technology, Cam APS system)[14,15] with lower target glucose levels and more robust algorithms can reduce postprandial hyperglycemia. The 5-minutely automated correction doses with 780G SmartGuard, the hourly corrections with Control-IQ, and the “Add meal” and/or “Boost mode” with Cam APS would be expected to reduce postprandial hyperglycemia from HPHF meals.
There were several limitations with this study, although this is the first study to investigate how the auto mode algorithm responds to HPHF meals in children and adolescents with T1D in a free-living environment, the small sample size resulted in a lack of power to detect all but the largest of effects with lack of statistically significant differences in all the glucose excursion outcome measures investigated between the auto mode and manual mode. Although HPHF meals are well known to cause large and delayed postprandial glucose excursions in individuals with T1D,[2-4] there are no evidence-based insulin-dosing guidelines for dealing with these meals in auto mode. This highlights the need for future nutrition-focused studies to develop guidelines for new diabetes management technologies. Future studies could also consider a follow-up period longer than 10 h as previous studies have noted HPHF meals can result in prolonged glucose excursions that can last for up to 10–12 h after eating.[2-5] Finally, although our interviews allowed the capture of real-life experiences about the two insulin delivery modes, the participants were almost all regular users of auto mode prior to the study, which limits our capacity to engage in valid comparisons between this mode of insulin delivery and the use of dual wave mode. It is vital to consider the impact of protein and fat and dietary behaviors of families with T1D, especially in free-living settings while designing studies on newer closed loop therapy systems.
CONCLUSION
In this pilot study, although most families felt confident with auto mode for postprandial HPHF excursions, this was not reflected in the postprandial glucose levels.
Author’s contributions
RJL, MBA, PAF and AJH contributed to the study design, data analysis, and interpretation of results. With assistance from Grant Smith, RJL analyzed the data and provided statistical advice. RJL, MBA, PAF, AJH wrote the manuscript. All authors critically reviewed the manuscript, contributed to the discussion, read, and approved the final version submitted for publication.
Acknowledgments
The authors would like to thank the families who participated in this study. We acknowledge the support from Biostatistician Grant Smith and Project Manager Heather Roby.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
The research team was supported by the JDRF Australian T1D Clinical Research Network (4-SRA-2015-157-MB), a special initiative of the Australian Research Council.
RJL was supported by a scholarship from the Children’s Diabetes Centre, a JDRF/National Health and Medical Research Council funded Centre of Research Excellence in T1D (APP1078190).
MBA was supported by the Department of Health/Raine Clinical Research Fellowship from Western Australia.
References
- Australian Health Survey: Nutrition First Results-Foods and Nutrients. 2011. Canberra: ABS; Available from: https://www.abs.gov.au/statistics/health/health-conditions-and-risks/australian-health-survey-nutrition-first-results-foods-and-nutrients/latest-release [Last accessed on 2022 Sep 30]
- [Google Scholar]
- Does meal-time insulin dosing based on fat-protein counting give positive results in postprandial glycaemic profile after a high protein-fat meal in adolescents with Type 1 diabetes: A randomised controlled trial. J Hum Nutr Diet. 2019;33:396-403.
- [CrossRef] [PubMed] [Google Scholar]
- Higher glucose concentrations following protein-and fat-rich meals-the tuebingen grill study: A pilot study in adolescents with Type 1 diabetes. Pediatr Diabetes. 2015;16:587-91.
- [CrossRef] [PubMed] [Google Scholar]
- Does the fat-protein meal increase postprandial glucose level in Type 1 diabetes patients on insulin pump: The conclusion of a randomized study. Diabetes Technol Ther. 2012;14:16-22.
- [CrossRef] [PubMed] [Google Scholar]
- Protein and fat meal content increase insulin requirement in children with Type 1 diabetes-role of duration of diabetes. J Clin Transl Endocrinol. 2017;10:15-21.
- [CrossRef] [PubMed] [Google Scholar]
- ISPAD Clinical Practice Consensus Guidelines 2022: Nutritional management in children and adolescents with diabetes. Pediatr Diabetes. 2022;23:1297-321.
- [CrossRef] [PubMed] [Google Scholar]
- Optimizing hybrid closed-loop therapy in adolescents and emerging adults using the MiniMed 670G system. Diabetes Care. 2018;41:798-6.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and efficacy of Medtronic MiniMed 670G hybrid closed-loop system in the diabetes camp setting: Comparison of auto mode (AM) vs manual mode (MM) Diabetes. 2019;68(S1):1075.
- [CrossRef] [Google Scholar]
- A randomized trial of closed-loop control in children with Type 1 diabetes. N Engl J Med. 2020;383:836-45.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of a hybrid closed-loop system on glycemic and psychosocial outcomes in children and adolescents with Type 1 diabetes: A randomized clinical trial. JAMA Pediatr. 2021;175:1227-35.
- [CrossRef] [PubMed] [Google Scholar]
- Extended use of the control-IQ closed-loop control system in children with Type 1 diabetes. Diabetes Care. 2021;44:473-8.
- [CrossRef] [PubMed] [Google Scholar]
- Patient satisfaction and clinical experience with MiniMed 670G hybrid closed-loop system. ADCES. 2020;8:28-32.
- [CrossRef] [Google Scholar]
- Optimizing a hybrid closed loop system in Type 1 diabetes: A case report. Diabetes Ther. 2018;9:2173-7.
- [CrossRef] [PubMed] [Google Scholar]
- A comparison of two hybrid closed-loop systems in adolescents and young adults with Type 1 diabetes (FLAIR): A multicentre, randomised, crossover trial. Lancet. 2021;397:208-19.
- [CrossRef] [PubMed] [Google Scholar]
- Six-month randomized, multicenter trial of closed-loop control in Type 1 diabetes. N Engl J Med. 2019;381:1707-17.
- [CrossRef] [PubMed] [Google Scholar]







