Translate this page into:
Early neurological association in a young child with Wolfram syndrome

*Corresponding author: Aashima Dabas, Department of Pediatrics, Maulana Azad Medical College, New Delhi, India. dr.aashimagupta@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kumar K, Dabas A, Saxena R. Early neurological association in a young child with Wolfram syndrome. J Pediatr Endocrinol Diabetes. 2023;3:118-9. doi: 10.25259/JPED_20_2023
Dear Editor,
Wolfram syndrome (WFS) is characterized by juvenile-onset diabetes mellitus (DM), diabetes insipidus, optic nerve atrophy, sensorineural hearing loss (SNHL), and neurodegeneration.[1,2] We report a child with WFS and early central nervous system (CNS) dysfunction presenting in the first decade of life.
A 10-year-old girl (index case), a known case of WFS with a family history of two other affected siblings, was diagnosed at 3 years of age with severe diabetic ketoacidosis. The patient was screened for central diabetes insipidus (CDI) at 8 years of age in view of recurrent urinary tract infections, renal tract abnormalities, and polyuria. CDI was confirmed on water deprivation testing, and she was commenced on oral desmopressin at 0.1 mg daily. The onset of CDI was earlier in the index case (8 years) than in the elder sibling at 10 years. The audiometric evaluation suggested bilateral SNHL at 3 years, which was earlier in onset than the other two siblings (at 9 and 5 years, respectively), accompanied by an earlier onset of optic atrophy at 7 years than others (at 9 and 9.5 years, respectively).
At 10 years of age, she presented to the pediatric emergency room with status epilepticus and gasping respiratory efforts. The child had an antecedent history of a seizure episode for more than 5 min, followed by loss of consciousness for 2 hours. Capillary dextrose at the onset of the event at home was 45 mg/dL, which was treated with glucose solution as informed by the mother. She denied any preceding illness or insulin administration errors. On examination, the sensorium was depressed (E1V1M2), with poor perfusion and shallow respiratory effort. Venous blood tests for the metabolic panel showed random blood glucose of 50 mg/dL, with normal calcium levels and electrolytes, glycated hemoglobin 9.5%, and cortisol 438 nmol/L (normal >420 nmol/L). The patient was intubated, stabilized, and managed for status epilepticus with intravenous lorazepam and sodium valproate. The patient was gradually weaned off ventilation in 48 h and extubated with improvement in sensorium. No repeat episode of hypoglycemia was documented during the hospital stay. There were no residual neurological deficits or signs.
A possibility of CNS dysfunction was considered as there was disproportionately severe neurological involvement for the degree of hypoglycemia. Magnetic resonance imaging of the brain performed after 7 days showed brainstem hypoplasia and cerebellar atrophy [Figure 1]. The child was discharged home on maintenance doses of sodium valproate (20 mg/kg/day) and has remained well and seizure-free till the last follow-up 6 months later.
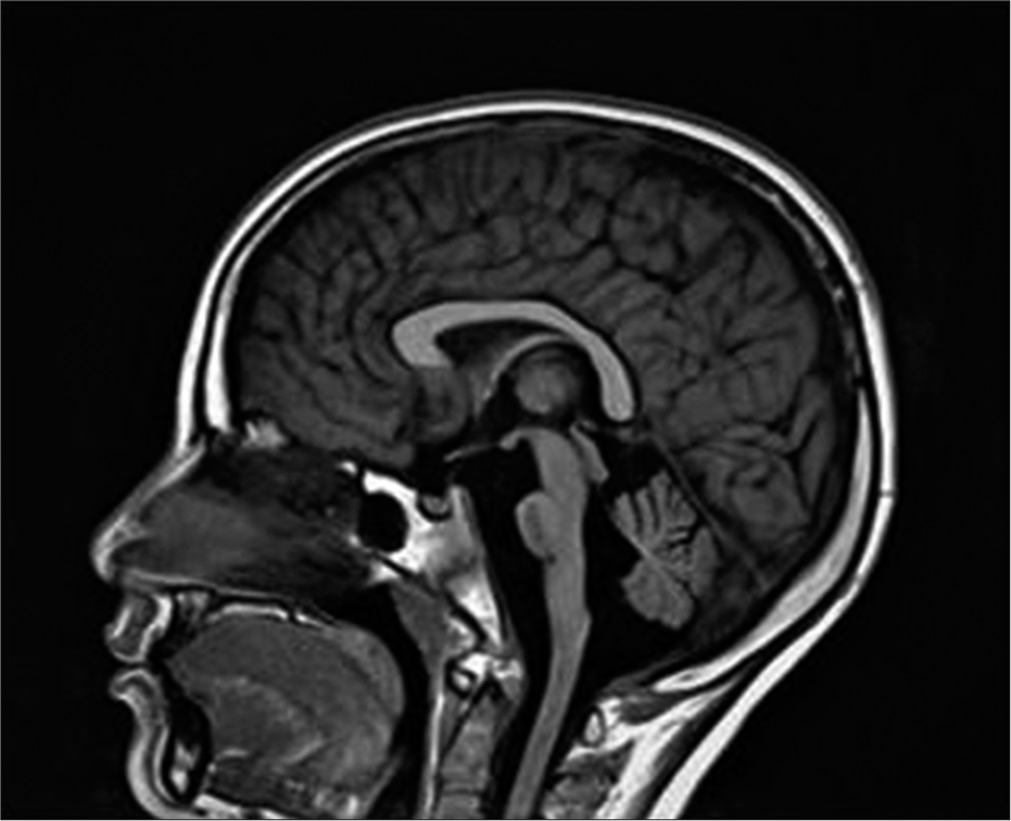
- Sagittal image of magnetic resonance imaging brain and brainstem showing brain stem and cerebellar hypoplasia with absent posterior pituitary bright spot.
Neurological complications involve 62% of WFS patients, appearing at an average age of 16 years (mean age 30 years).[2,3] Cerebellar ataxia is one of the most common neurologic complications. Other signs are dysarthria, dysphagia, areflexia, epilepsy (absence, myoclonic epilepsy, or tonic-clonic seizures), nystagmus, and anosmia. The index patient had an earlier onset of epilepsy and involvement of the brainstem and cerebellum. Common causes of mortality are respiratory failure due to central apnea and dysphagia leading to aspiration pneumonitis as a consequence of brain stem atrophy [3] that will require close monitoring in the index case. The preferential vulnerability of the brainstem and cerebellum in WFS has been described previously, generally in adults in advanced disease states, without quantification or control groups. However, Hershey et al.[4] showed brainstem and cerebellum volume abnormalities even with the initial non-neurological manifestations of WFS. In addition to the profound subcortical and cerebellar changes, cortical thickness is also reduced in WFS in restricted regions, including lingual, precentral, and rostral middle frontal cortex.[3]
The occurrence of hypoglycemic seizures is not uncommon in children with DM. A low threshold should be kept for evaluating children for sinister associations in children with syndromic diabetes to avoid missing neurological causes like brainstem hypoplasia.
At present, there are no effective measures to prevent disease from progression; however, early recognition of dangerous signs and instituting symptomatic treatment and rehabilitative measures can improve the quality of life. The complex nature of the disease with life-threatening emergencies necessitates the formulation of an emergency action and escalation plan on discussion between family and healthcare providers. The authors are reporting this case to highlight the possibility of early neurological involvement as a part of multisystemic involvement in syndromic diabetes, such as WFS and the need for neuroimaging if there are neurological features disproportionate to the degree of hypoglycemia.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Wolfram's syndrome: A clinical, diagnostic, and interpretative contribution. Diabetes Care. 1986;9:521-8.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical characteristics of Wolfram syndrome in Chinese population and a novel frameshift mutation in WFS1. Front Endocrinol (Lausanne). 2018;9:18.
- [CrossRef] [PubMed] [Google Scholar]
- Neurodegeneration and diabetes: UK nationwide study of Wolfram (DIDMOAD) syndrome. Lancet. 1995;346:1458-63.
- [CrossRef] [PubMed] [Google Scholar]
- Early brain vulnerability in Wolfram syndrome. PLoS One. 2012;7:e40604.
- [CrossRef] [PubMed] [Google Scholar]





