Translate this page into:
Diabetic striatopathy – A rare manifestation of type 1 diabetes in children – A case report

*Corresponding author: Jijesh Alarambil, Assistant Professor, Department of Paediatrics, Government Medical College, Kannur, Kerala, India. jijeshalarambil@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Gopinath R, Alarambil J. Diabetic striatopathy – A rare manifestation of type 1 diabetes in children – A case report. J Pediatr Endocrinol Diabetes. 2024;4:98-100. doi: 10.25259/JPED_10_2024
Abstract
Diabetic striatopathy is a complication of poorly controlled diabetes mellitus and is uncommon in children. This condition manifests as movement disorders, with the most common being hemichorea and hemiballismus. A girl with long-standing poorly controlled diabetes presenting as hemiballismus is being reported.
Keywords
Type 1 diabetes
Extrapyramidal
Hemichorea-hemiballismus
Childhood diabetic striatopathy
INTRODUCTION
Diabetic striatopathy, also known as “hyperglycemic non-ketotic hemichorea/hemiballism,” is a known neurological complication of diabetes, usually seen in the adult population with low incidence in children.[1] It manifests as acute onset abnormal body movements such as hemichorea or hemiballismus and is generally associated with poor glycemic control. Neuroimaging shows characteristic findings that aid in diagnosis.
CASE REPORT
A 14-year-old girl, a known case of type 1 diabetes mellitus, presented with intermittent choreiform movements of her right upper limb, which progressed to involve the right lower limb in 2 days. The movements were present even at rest, were exacerbated by physical activity, and disappeared during sleep. Due to these movements, there was pain and uneasiness in the limb. There was no history of loss of consciousness, fever, or headache during or preceding these complaints. There was no history of drug intake besides insulin. She was diagnosed with type 1 diabetes at the age of 8 years and had been on treatment with insulin since then, initially on premixed insulin and later changed to a basal-bolus regime with glargine 15 IU and regular insulin 30 IU in divided doses. Compliance with treatment was very poor, and she had poorly controlled diabetes with an elevated HbA1C of 14%. Her parents had separated, and she had many admissions with diabetic ketoacidosis several times in the past. There was no history of measles like illness in her childhood.
At admission, the child was conscious and oriented. She had low amplitude choreiform and occasional ballismic movements involving the right upper and lower limbs without facial involvement. She did not have seizures or any extrapyramidal features such as rigidity, tremors, or bradykinesia. Examination of the nervous system revealed normal cranial nerves, sensory, and motor systems. No Kayser Fleischer ring was present in slit-lamp examination. The speech was clear, though she appeared depressed and tired.
Blood examination revealed a random blood glucose of 400 mg/dL with normal venous blood gas and absent urine ketones. Blood routine assessments, serum calcium, and serum magnesium were within normal limits. Serum ceruloplasmin and 24 h urinary copper excretion were also within normal limits. The electroencephalogram was normal. A diagnosis of uncontrolled diabetes without ketoacidosis was made. Other investigations included serum sodium–132 meq/dL, serum potassium–4 meq/ dL, and serum antistreptolysin O titer <200 mIU/L. Brain magnetic resonance imaging (MRI) (Siemens Avanto 1.5 T; Siemens Healthcare USA, Malvern, PA) was performed with classic sequences (SE T1, SE T2, fluid-attenuated inversion recovery, susceptibility-weighted imaging, and diffusion-weighted imaging) and it showed patchy T1 hyperintense and T2 hypointense areas of signal alteration predominantly involving caudate, globus, and anterior putamen left > right [Figure 1]. The child was further screened for diabetic nephropathy and retinopathy, but no significant findings were noted.
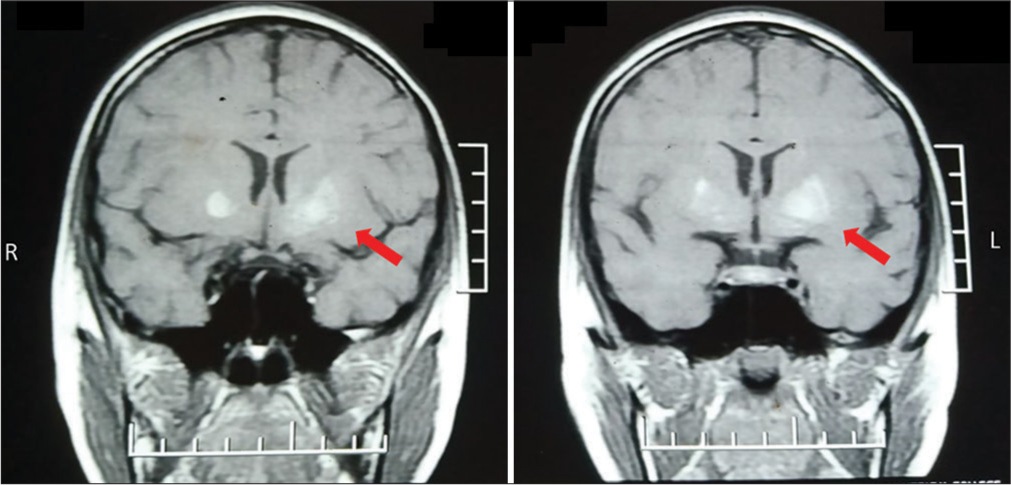
- Coronal T1-weighted magnetic resonance imaging of that child, showing the diffuse high intensity of the bilateral putamen and globus pallidus which was more intense on left than on the right. Red arrows showing left putamen.
She improved symptomatically with strict glycemic control with insulin and risperidone 0.5 mg daily therapy as a first-line agent and continued for 6 months after seeking neurology opinion.
DISCUSSION
Pediatric diabetic striatopathy presenting as extrapyramidal manifestation is attributed to poor glycemic control. The etiology of this condition is hypothesized to be due to the metabolic changes and vascular pathology caused by chronic hyperglycemia; both together contribute to the clinical manifestations.[2] In non-ketotic hyperglycemia, brain metabolism is shifted toward the alternative anaerobic pathway in the Krebs cycle, causing depletion in gamma-aminobutyric acid (GABA), an inhibitory neurotransmitter. This leads to attenuated inhibition of the subthalamus by the medial globus pallidus, resulting in hyperkinetic movements.[3,4] The hyperviscosity state caused by chronic hyperglycemia and diabetic vasculopathy further leads to thrombus formation and regional ischemia.
Clinically, striatal involvement manifests as hemichorea and hemiballismus and is usually unilateral. Weakness or paresthesia, behavioral abnormalities, seizures, and altered level of consciousness are reported preceding hemichorea and hemiballismus. Our patient had unilateral symptoms even though neuroimaging showed bilateral involvement. Usual radiological findings in this condition include high attenuation in the contralateral caudate nucleus and putamen in computed tomography and high T1 signal in the affected striatum in MRI imaging.
Treatment includes strict glycemic control and correction of dehydration, along with anti-chorea medications. Four categories of drugs – antipsychotics, GABA receptor antagonists, selective serotonin reuptake inhibitors, and dopamine-depleting agents can be used as anti-chorea medications. Even though the exact duration of treatment is not known, symptoms usually resolve within 2–28 days.[5,6]
CONCLUSION
Neurocomplications, though rare, can occur in poorly controlled diabetes in the pediatric age group. Close monitoring and strict glycemic control will help to prevent these complications and will improve their quality of life.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Diabetic striatal disease: Clinical presentation, neuroimaging, and pathology. Intern Med. 2009;48:1135-41.
- [CrossRef] [Google Scholar]
- Diabetic striatopathy In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, eds. Endotext. South Dartmouth, MA: MDText.com, Inc.; 2000.
- [Google Scholar]
- Movement disorders in patients with diabetes mellitus. J Neurol Sci. 2012;314:5-11.
- [CrossRef] [Google Scholar]
- "Diabetic striatopathy" and ketoacidosis: Report of two cases and review of literature. Diabetes Res Clin Pract. 2017;128:1-5.
- [CrossRef] [Google Scholar]
- Diabetic striatopathy: A new challenge in type 1 pediatric diabetic patients. Oman Med J. 2022;37:e332.
- [CrossRef] [Google Scholar]






